28898-02-4
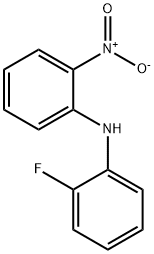 28898-02-4 结构式
28898-02-4 结构式
基本信息
2-氟-2'-硝基二苯基胺
2-氟-N-(2-硝基苯基)苯胺
N-(2-fluorophenyl)-2-nitroaniline
2-Fluoro-N-(2-nitrophenyl)aniline
2-[(2-Fluorophenyl)amino]nitrobenzene
Benzenamine, 2-fluoro-N-(2-nitrophenyl)-
2-[(2-Fluorophenyl)amino]nitrobenzene 98%
2-FLUORO-2'-NITRODIPHENYLAMINE ISO 9001:2015 REACH
2-Fluoro-N-(2-nitrophenyl)aniline, 2-Fluoro-2'-nitrodiphenylamine
制备方法
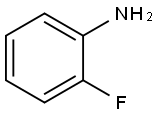
348-54-9
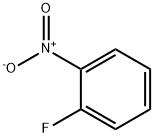
1493-27-2

28898-02-4
通用方法:在0℃下,将NaH(75 mmol)悬浮于DMF(60 mL)中,缓慢加入邻氟苯胺(50 mmol)。保持0℃并搅拌30分钟后,逐滴加入1-氟-2-硝基苯(60 mmol)的DMF(30 mL)溶液。将反应混合物逐渐升温至室温并持续搅拌16小时。反应完成后,将混合物小心倒入搅拌的饱和NH4Cl溶液(500 mL)中淬灭反应。过滤收集沉淀,用水洗涤滤饼,最后通过甲醇重结晶纯化,得到目标产物2-氟-N-(2-硝基苯基)苯胺(15j)。产物为红色固体,产率74.1%,熔点74.4-75.8℃。1H NMR (400 MHz, DMSO-d6) δ 9.28 (s, 1H), 8.14 (d, J = 8.0 Hz, 1H), 7.51 (m, 2H), 7.35 (m, 3H), 6.90 (m, 2H); MS (ESI) m/z (%): 231.1 [M-H]-。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2017, vol. 25, # 16, p. 4475 - 4486
[2] Molecules, 2015, vol. 20, # 1, p. 1712 - 1730
[3] Archiv der Pharmazie, 1997, vol. 330, # 11, p. 353 - 357
[4] Patent: WO2008/73459, 2008, A1. Location in patent: Page/Page column 95
[5] Journal of Medicinal Chemistry, 2010, vol. 53, # 11, p. 4511 - 4521
