292150-20-0
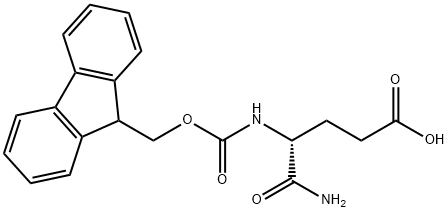 292150-20-0 结构式
292150-20-0 结构式
基本信息
FMOC-ALPHA-谷氨酸盐
(4R)-5-氨基-4-[[芴甲氧羰基]氨基]-5-氧代戊酸
(R)-4-((((9H-芴-9-基)甲氧基)羰基)氨基)-5-氨基-5-氧戊二酸
Fmoc-D-Igln-OH
Fmoc-DL-Glu-NH2
Fmoc-d-isoglutamine
FMOC-alpha-glutaMine
N-Fmoc-D-isoglutamine
Fmoc-alpha-Glutamine, >97%
Fmoc-D-glutamic acid α-amide
FMOC-alpha-glutaMine USP/EP/BP
FMOC-D-ISOGLN-OH(FMOC-D-GLU-NH2)
制备方法
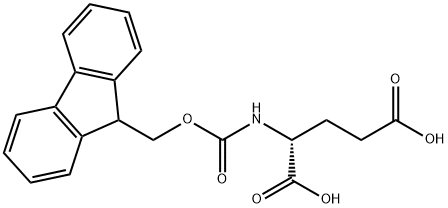
104091-09-0

292150-20-0
以(R)-2-((((9H-芴-9-基)甲氧基)羰基)氨基)戊二酸为原料合成(R)-4-((((9H-芴-9-基)甲氧基)羰基)氨基)-5-氨基-5-氧戊二酸的一般步骤:将Fmoc-D-Glu-OH(59.8 g,1.0当量)溶解于无水四氢呋喃(THF,324 mL)中。在冰水浴冷却下搅拌,缓慢加入N,N'-二环己基碳二亚胺(DCC,40.1 g,1.2当量)。将反应混合物逐渐升温至室温,继续搅拌8小时,生成1,3-二环己基脲(DCU)沉淀。过滤去除沉淀,并用少量THF洗涤。随后,在NaCl-冰浴冷却下,向滤液中通入干燥氨气,持续搅拌1.5小时,直至无白色固体析出。静置30分钟后,加入少量甲醇(MeOH)以溶解固体。再次将混合物置于冰水浴中冷却,缓慢滴加2.0 N HCl溶液调节pH至2-3。减压浓缩去除溶剂。将所得固体溶于乙酸乙酯(AcOEt)中,依次用稀HCl、饱和NaHCO3水溶液和去离子水洗涤。分离有机层,合并后用无水硫酸镁(MgSO4)干燥过夜。过滤后,减压蒸发溶剂,残余物通过乙酸乙酯-环己烷体系重结晶。经过滤,得到46.5 g目标产物,产率78%。产物熔点为204-205℃,比旋光度[α] = -4.2°(c = 10 mg/mL,DMF)。1H-NMR(500 MHz,DMSO-d6)δ:7.88(2H,d,J = 8.0 Hz),7.72(2H,m),7.42(2H,m),7.40(1H,m),7.40(1H,br.s),7.32(2H,m),7.02(1H,br.s),4.27(2H,m),4.20(1H,m),3.93(1H,dd,J = 13.5,8.5 Hz),2.25(2H,m),1.89(1H,m),1.73(1H,m)。13C-NMR(125 MHz,DMSO-d6)δ:173.9,173.4,155.9,143.8,140.7,127.6,127.0,125.3,120.0,65.6,53.8,46.6,30.4,27.2。ESI-MS:m/z 369.03 [M + H]+,759.98 [2M + Na]+。HR-MS(TOF):m/z 369.1448 [M + H]+(计算值:369.1449),759.2623 [2M + Na]+(计算值:759.2625),分子式C20H20N2O5。
参考文献:
[1] Patent: EP2612857, 2017, B1. Location in patent: Paragraph 0102-0107