29509-06-6
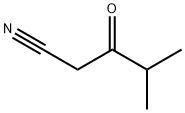 29509-06-6 结构式
29509-06-6 结构式
基本信息
4-甲基-3-氧代戊腈
Isobutyrylacetonitrile
4-methyl-3-oxovaleronitrile
Isopropyl cyanomethylketone
4-METHYL-3-OXOPENTANENITRILE
3-Oxo-4-methylpentanenitrile
Pentanenitrile, 4-methyl-3-oxo-
4-Methyl-3-oxopentanenitrile 97+%
Isobutanoylacetonitrile, 4-Methyl-3-oxovaleronitrile
Isobutanoylacetonitrile, 1-Cyano-3-methylbutan-2-one
物理化学性质
制备方法

75-05-8
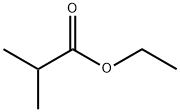
97-62-1

29509-06-6
通用方法:将异丁酸乙酯(6.65 mmol,1当量)在室温下以约230 rpm的转速搅拌溶解于THF(30 mL,工业级,含水0.2%)中,持续5分钟。随后,迅速向上述THF溶液中加入叔丁醇钾(1.57 g,14.0 mmol,95%,2当量)。充分搅拌反应瓶后,加入乙腈(6.65 mmol,1当量)。将所得混合物在室温下继续搅拌。反应完成后,通过加入水(50 mL)淬灭反应,继续搅拌5分钟。接着,加入乙酸乙酯(40 mL)和HCl溶液(1 mL,12 M),分离有机层,并用无水硫酸镁干燥。减压蒸发去除溶剂,将残余物加载至开放式硅胶柱顶部(柱尺寸:3×15 cm,洗脱剂为正己烷/乙酸乙酯(3:1,v/v);对于特定产物,柱尺寸调整为3.5×8 cm,洗脱剂为CH2Cl2)。收集含目标产物3-氧代-4-甲基戊腈的馏分,减压蒸发后得到纯化的β-酮腈产物。
参考文献:
[1] Tetrahedron, 2013, vol. 69, # 48, p. 10331 - 10336
[2] Patent: CN104059024, 2016, B. Location in patent: Paragraph 0061-0064
[3] Organic Letters, 2006, vol. 8, # 6, p. 1161 - 1163
[4] Patent: WO2010/42684, 2010, A1. Location in patent: Page/Page column 57-58
[5] Chemische Berichte, 1982, vol. 115, # 1, p. 355 - 380
