29913-51-7
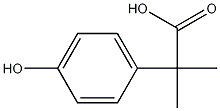 29913-51-7 结构式
29913-51-7 结构式
基本信息
4-羟基-.ALPHA.,.ALPHA.-二甲基-苯乙酸
4-Hydroxy-α,α-dimethylbenzeneacetic acid
4-Hydroxy-α,α-dimethyl-benzeneacetic acid
2-(4-Hydroxyphenyl)-2-Methylpropanoic acid
Benzeneacetic acid, 4-hydroxy-α,α-diMethyl-
Benzeneacetic acid, 4-hydroxy-alpha,alpha-diMethyl-
4-hydroxy-.alpha.,.alpha.-dimethyl-Benzeneacetic acid
物理化学性质
制备方法
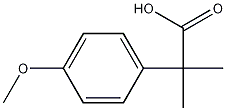
2955-46-6

29913-51-7
步骤24c:2-(4-羟基苯基)-2-甲基丙酸(化合物0804-27)的合成 将2-(4-甲氧基苯基)-2-甲基丙酸(化合物0803-27)(0.85g,4.38mmol)与吡啶盐酸盐(2.82g,24.40mmol)混合,于180℃加热反应5小时。反应完成后,将混合物冷却至室温,用稀盐酸(1M)调节pH至6,随后用乙酸乙酯进行萃取。分离有机层,用无水硫酸钠干燥,浓缩后得到目标产物0804-27(0.61g,收率76%),为无色油状物。LCMS检测显示m/z为181 [M + 1]+。1H NMR(400MHz,DMSO-d6)数据如下:δ1.41(s,6H),6.70(d,J = 8.8Hz,2H),7.13(d,J = 8.8Hz,2H),9.28(s,1H),12.16(s,1H)。
参考文献:
[1] Journal of Medicinal Chemistry, 1994, vol. 37, # 26, p. 4508 - 4521
[2] Patent: WO2005/121102, 2005, A2. Location in patent: Page/Page column 25-26
[3] Patent: WO2009/36016, 2009, A1. Location in patent: Page/Page column 78c
[4] Bioorganic and Medicinal Chemistry Letters, 2006, vol. 16, # 17, p. 4567 - 4570
[5] Journal of Medicinal Chemistry, 2010, vol. 53, # 16, p. 6129 - 6152