30189-48-1
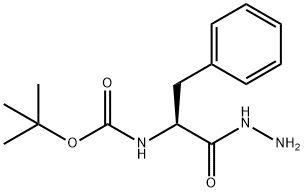 30189-48-1 结构式
30189-48-1 结构式
基本信息
N-(1-苄基-2-肼基-2-氧乙基)氨基甲酸叔丁酯
(S)-(1-肼基-1-氧代-3-苯基丙-2-基)氨基甲酸叔丁酯
(S)-叔丁基(1-肼基-1-氧代-3-苯基丙-2-基)氨基甲酸酯
N-(TERT-BUTOXYCARBONYL)-L-PHENYLALANINE ACID HYDRAZIDE
TERT-BUTYL N-(1-BENZYL-2-HYDRAZINO-2-OXOETHYL)CARBAMATE
(2S)-2-[(tert-Butoxycarbonyl)amino]-3-phenylpropanohydrazide
L-Phenylalanine, N-[(1,1-dimethylethoxy)carbonyl]-, hydrazide
Tert-Butyl N-(1-Hydrazinyl-1-Oxo-3-PhenylpropaN-2-Yl)Carbamate
(S)-tert-Butyl (1-hydrazinyl-1-oxo-3-phenylpropan-2-yl)carbamate
物理化学性质
制备方法
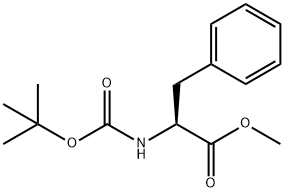
51987-73-6

30189-48-1
以N-叔丁氧羰基-L-苯丙氨酸甲酯为原料合成(S)-(1-肼基-1-氧代-3-苯基丙-2-基)氨基甲酸叔丁酯的一般步骤:向N-叔丁氧羰基-L-苯丙氨酸甲酯(300 mg,1.07 mmol)的甲醇(5.4 mL)溶液中加入水合肼(170 μL,3.44 mmol)。将反应混合物在室温下搅拌过夜。反应完成后,通过旋转蒸发仪将溶剂减压浓缩。所得残余物先用己烷(2×)研磨,再用最小体积的2:3己烷/甲醇混合溶剂(2×)研磨,得到白色固体状目标产物(S)-(1-肼基-1-氧代-3-苯基丙-2-基)氨基甲酸叔丁酯(195 mg,收率65%)。产物结构经1H NMR(CDCl3)δ 7.41-7.25(m, 3H),7.20(d, J = 6.8 Hz, 2H),4.39-4.21(m, 1H),3.07(d, J = 7.0 Hz, 2H),1.42(s, 9H)及质谱(ESI+)m/z 280.3([M+H]+)确认。
参考文献:
[1] New Journal of Chemistry, 2018, vol. 42, # 6, p. 4344 - 4351
[2] Journal of Organic Chemistry, 1995, vol. 60, # 10, p. 3112 - 3120
[3] Angewandte Chemie - International Edition, 2016, vol. 55, # 32, p. 9422 - 9426
[4] Angew. Chem., 2016, vol. 55, # 32, p. 9569 - 9574,6
[5] Patent: WO2013/192286, 2013, A1. Location in patent: Paragraph 00281
