313369-26-5
 313369-26-5 结构式
313369-26-5 结构式
基本信息
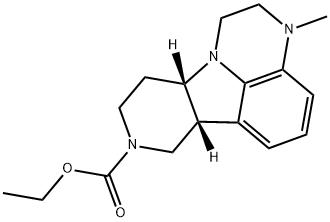
乙基(6BR,10AS)-3-甲基-2,3,6B,9,10,10A-六氢-1H-吡啶并[3',4':4,5]吡咯并[1,2,3-DE]喹喔啉-8(7H)-羧酸盐
(6bR,10aS)-Ethyl 3-methyl-2,3,6b,7,10,10a-hexahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(9H)-carboxylate
Ethyl (6bR,10aS)-3-methyl-2,3,6b,9,10,10a-hexahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(7H)-carboxylate
1H-Pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(7H)-carboxylic acid, 2,3,6b,9,10,10a-hexahydro-3-methyl-, ethyl ester, (6bR,10aS)-
物理化学性质
制备方法
313369-25-4
![(6bR,10aS)-3-甲基-2,3,6b,7,10,10a-六氢-1H-吡啶并[3',4':4,5]吡咯并[1,2,3-de]喹喔啉-8(9H)-羧酸叔丁酯](/CAS/20180601/GIF/313369-26-5.gif)
313369-26-5
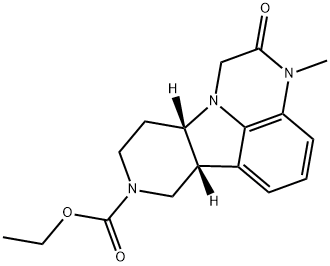
步骤CA:在氮气保护下,将硼烷四氢呋喃溶液(1M,33mL,33mmol)缓慢滴加至搅拌中的(6bR,10aS)-3-甲基-2-氧代-2,3,6b,7,10,10a-六氢-1H-吡啶并[3',4':4,5]吡咯并[1,2,3-de]喹喔啉-8(9H)-羧酸叔丁酯(5.24g,16.6mmol)的无水四氢呋喃(25mL)溶液中。滴加完毕后,将反应混合物加热回流1小时。冷却后,加入6N盐酸(15mL),继续在回流条件下加热30分钟。反应完成后,冷却混合物并在减压下蒸发至干。将残余物溶解于最小量的水中,用1N氢氧化钠溶液碱化后,用二氯甲烷(2×)萃取。合并有机相,用水洗涤,硫酸镁干燥,浓缩后得到4.65g(93%收率)的(6bR,10aS)-3-甲基-2,3,6b,7,10,10a-六氢-1H-吡啶并[3',4':4,5]吡咯并[1,2,3-de]喹喔啉-8(9H)-羧酸叔丁酯,为粘稠液体。1H NMR (CDCl3, 300MHz) δ 1.28 (t, J = 7Hz, 3H), 1.68-1.78 (m, 1H), 1.78-1.93 (m, 2H), 2.81-2.90 (m, 2H), 2.86 (s, 3H), 3.05-3.26 (m, 2H), 3.26-3.38 (m, 2H), 3.56-3.75 (m, 2H), 3.79-3.87 (m, 1H), 4.16 (q, J = 7Hz, 2H), 6.41 (d, J = 8.1Hz, 1H), 6.61 (d, J = 8.1Hz, 1H), 6.67 (t, J = 8.1Hz, 1H) ppm。MS (CI): 302 (M + H+)。
参考文献:
[1] Patent: US2004/220178, 2004, A1
[2] Patent: WO2008/112280, 2008, A1. Location in patent: Page/Page column 90-91
[3] Patent: WO2008/112280, 2008, A1. Location in patent: Page/Page column 90-91