330785-81-4
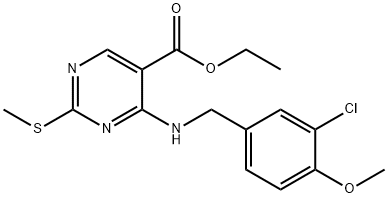 330785-81-4 结构式
330785-81-4 结构式
基本信息
阿伐那非杂质P
AVANAFIL杂质P
2-甲巯基-4-(3-氯-4-甲氧基苄氨基)嘧
4-3-氯-4-甲氧基苄胺基-2-甲巯基-5-乙氧羰基嘧啶
4-(3-氯-4-甲氧基苄胺)-5-甲酸乙酯-2-甲硫基嘧啶
2-甲硫基-4-(3-氯-4-甲氧基苄胺) -5-羧酸乙酯嘧啶
2-甲硫基-4-(3-氯-4-甲氧基苄胺基)嘧啶-5-羧酸乙酯
4-(3-氯-4-甲氧基苄氨基)-2-甲巯基-5-乙氧羰基嘧啶
4-(3-氯-4-甲氧基苯氨基)-5-乙氧基羰基-2-甲基噻嘧啶
INTERMEDIATE OF AVANAFIL
4-(3-chloro-4-MethoxybenzylaMino)-5-ethoxycarbonyl-2-MethylthiopyriMidine
ethyl 4-(3-chloro-4-MethoxybenzylaMino)-2-(Methylthio)pyriMidine-5-carboxylate
ethyl 4-[(3-chloro-4-methoxyphenyl)methylamino]-2-methylsulfanylpyrimidine-5-carboxylate
4-(3-Chloro-4-Methoxy-benzylaMino)-2-Methylsulfanyl-pyriMidine-5-carboxylic acid ethyl ester
4-[[(3-Chloro-4-methoxyphenyl)methyl]amino]-2-(methylthio)-5-pyrimidinecarboxylic acid ethyl ester
(5-PyriMidinecarboxylicacid, 4-[[(3-chloro-4-Methoxyphenyl)Methyl]aMino]-2-(Methylthio)-,ethyl ester)
(5-PyriMidinecarboxylicacid, 4-[[(3-chloro-4-Methoxyphenyl)Methyl]aMino]-2-(Methylthio)-,ethyl ester)###NA
Avanafil intermediates,5-Pyrimidinecarboxylicacid, 4-[[(3- chloro-4- methoxyphenyl)methyl]amino]-2- (methylthio)-, ethyl ester
物理化学性质
制备方法
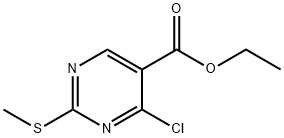
5909-24-0
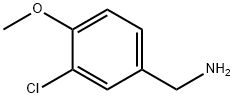
115514-77-7

330785-81-4
以4-氯-2-甲硫基嘧啶-5-羧酸乙酯和3-氯-4-甲氧基苄胺为原料合成4-(3-氯-4-甲氧基苄胺基)-2-甲巯基-5-乙氧羰基嘧啶的一般步骤:首先,将3-氯-4-甲氧基苄胺(45.6g,0.27mol)溶解于180mL丙酮中,随后加入三乙胺(40.4g,0.4mol)。接着,将4-氯-2-甲硫基嘧啶-5-羧酸乙酯(53.3g,0.24mol)溶于250mL丙酮中,缓慢滴加至上述反应溶液中。反应在室温下进行3小时。反应完成后,将反应液倒入冰水混合物中,用乙酸乙酯萃取。合并有机相,用10%柠檬酸水溶液(250mL×3)洗涤。有机层用无水硫酸钠干燥,随后减压蒸发溶剂。最后,产物经真空干燥,得到白色固体(86.0g,收率87.0%)。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2014, vol. 24, # 23, p. 5460 - 5465
[2] Bioorganic and Medicinal Chemistry Letters, 2015, vol. 25, # 7, p. 1431 - 1435
[3] Patent: CN108707141, 2018, A. Location in patent: Paragraph 0027; 0031; 0036; 0040; 0045; 0049
[4] Patent: EP2886540, 2015, A1. Location in patent: Paragraph 0168; 0169
[5] Patent: EP1366760, 2003, A1. Location in patent: Page 19
