33985-71-6
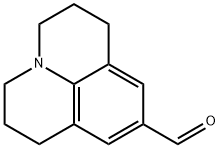 33985-71-6 结构式
33985-71-6 结构式
基本信息
9-醛基久洛尼定
2,3,6,7-四氢-1H,5H-苯并[ij]喹嗪-9-甲醛
1,2,3,5,6,7-六氢吡啶并[3,2,1-IJ]喹啉-9-甲醛
2,3,6,7-四氢-1H,5H-吡啶并[3,2,1-IJ]喹啉-9-甲醛
2,3,6,7-Tetrahydro-1
9-Julolidinecarboxaldehyde
9-Julolidinecarboxaldehyde>
2,3,3a,4,5,6-Hexahydro-3a-aza-1H-phenalene-8-carbaldehyde
1,2,3,4,5,6-Hexahydro-3a-aza-3aH-phenalene-8-carbaldehyde
2,3,6,7-Tetrahydro-1H,5H-benzo[ij]quinolizine-9-carbaldehyde
1,2,3,5,6,7-Hexahydropyrido[3,2,1-ij]quinoline-9-carbaldehyde
2,3,6,7-Tetrahydro-1H,5H-benzo[ij]quinolizine-9-carboxaldehyde
5H-BENZO(IJ)QUINOLIZINE-9-CARBOXALDEHYDE, 2,3,6,7-TETRAHYDRO-1
物理化学性质
制备方法
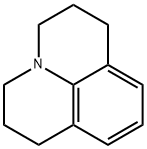
479-59-4

68-12-2

33985-71-6
将久洛尼定(0.5 g,2.89 mmol)、N,N-二甲基甲酰胺(0.255 g,3.49 mmol)和三氯氧磷(0.535 g,3.49 mmol)溶解于二氯甲烷(15 mL)中,混合物在氩气保护下于室温搅拌4小时。反应过程中溶液颜色变为绿色,反应进程通过薄层色谱(TLC)监测。反应完成后,用水淬灭反应,随后用2M氢氧化钠溶液和乙醚萃取粗产物。有机相经两次水洗后,用无水硫酸镁干燥,过滤并减压浓缩。粗产物通过柱色谱法纯化,洗脱剂为40%-50%乙醚/己烷,得到0.48 g(收率83.08%)的1,2,3,5,6,7-六氢吡啶并[3,2,1-ij]喹啉-9-甲醛,为浅黄色固体(当使用1.5 g久洛尼定时,收率可达92%)。产物结构经1H NMR(300 MHz, CDCl3)确认:δ 9.597(s, 1H),7.29(s, 1H),3.308(t, J = 57 Hz, 4H),2.787(t, J = 62 Hz, 4H),2.002-1.92(m, J = 63 Hz, 4H)。
参考文献:
[1] Tetrahedron, 2007, vol. 63, # 1, p. 103 - 114
[2] Organic Preparations and Procedures International, 2001, vol. 33, # 6, p. 603 - 613
[3] Journal of Nanoscience and Nanotechnology, 2015, vol. 15, # 11, p. 8813 - 8819
[4] Chemistry - A European Journal, 2010, vol. 16, # 30, p. 9257 - 9263
[5] Chemical Communications, 2014, vol. 50, # 78, p. 11507 - 11510
