35335-16-1
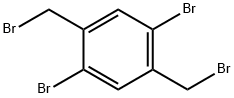 35335-16-1 结构式
35335-16-1 结构式
基本信息
1,4-DIBROMO-2,5-BIS(BROMMETHYL)BENZENE
1,4-Dibromo-2,5-bis(bromomethyl)benzene
2,5-Bis(Bromomethyl)-1,4-dibromobenzene
4-(Bromomethyl)-2,5-dibromobenzyl bromide
1,4-Bis(bromomethyl)-2,5-dibromobenzene97%
Benzene, 1,4-dibromo-2,5-bis(bromomethyl)-
1,4-Dibromo-2,5-bis(bromomethyl)benzene>
1,4-Bis(bromomethyl)-2,5-dibromobenzene 97%
2,5-Bis(bromomethyl)-1,4-dibromobenzene 97%
制备方法
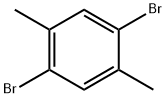
1074-24-4

35335-16-1
以2,5-二溴-1,4-二甲基苯为原料合成1,4-二溴-2,5-双(溴甲基)苯的一般步骤:将2,5-二溴-对二甲苯(26.4 g,0.1 mol)和N-溴代琥珀酰亚胺(NBS,39 g,0.22 mol)悬浮于四氯化碳(300 mL)中,加入过氧化苯甲酰(0.6 g)作为引发剂。在反应开始前,通入氮气流鼓泡5分钟以排除氧气。随后,将反应混合物置于100℃的油浴中加热回流2小时。反应完成后,加入乙醇(200 mL)以沉淀产物,过滤收集固体。用乙醇(50 mL)洗涤固体以去除残余的反应物和副产物,最后在真空下干燥,得到1,4-二溴-2,5-双(溴甲基)苯,为白色固体(13.36 g,产率31.6%)。产物结构经1H-NMR(500 MHz,CDCl3)确认:δ7.68(s,2H,芳环氢),4.50(s,4H,溴甲基氢)。
参考文献:
[1] Tetrahedron Letters, 2007, vol. 48, # 35, p. 6075 - 6079
[2] Organic and Biomolecular Chemistry, 2010, vol. 8, # 24, p. 5620 - 5627
[3] Journal of the American Chemical Society, 2017, vol. 139, # 45, p. 16210 - 16221
[4] Journal of the American Chemical Society, 2017, vol. 139, # 47, p. 17082 - 17088
[5] Journal of the American Chemical Society, 2009, vol. 131, # 49, p. 17724 - 17725
