356058-42-9
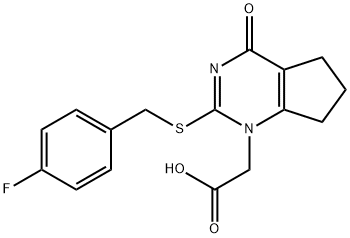 356058-42-9 结构式
356058-42-9 结构式
基本信息
2-(2 - ((4-氟苄基)硫基)-4-氧代-4,5,6,7-四氢-1H-环戊二烯并[D]嘧啶-1
2-(2-((4-氟苄基)硫基)-4-氧代-4,5,6,7-四氢-1H-环戊二烯并[D]嘧啶-1-基)乙酸
2-(2-((4-氟苯甲基)硫代)-4-氧亚基-4,5,6,7-四氢-1H-环戊二烯并[D]嘧啶-1-基)乙酸
2-[(4-Fluorobenzyl)thio]-4-oxo-4,5,6,7-tetrahydro-1H-cyclopenta[d]pyrimidine-1-acetic Acid
2-(2-((4-Fluorobenzyl)thio)-4-oxo-4,5,6,7-tetrahydro-1H-cyclopenta[d]pyrimidin-1-yl)acetic acid
1H-CyclopentapyriMidine-1-acetic acid, 2-[[(4-fluorophenyl)Methyl]thio]-4,5,6,7-tetrahydro-4-oxo-
2-[2-[(4-fluorophenyl)methylsulfanyl]-4-oxo-6,7-dihydro-5H-cyclopenta[d]pyrimidin-1-yl]acetic acid
制备方法
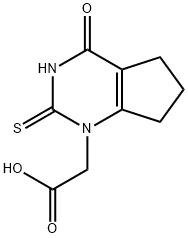
1349902-20-0
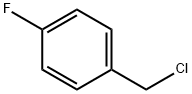
352-11-4
![2-(2 - ((4-氟苄基)硫基)-4-氧代-4,5,6,7-四氢-1H-环戊二烯并[D]嘧啶-1](/CAS/20180713/GIF/356058-42-9.gif)
356058-42-9
以2,3,4,5,6,7-六氢-4-氧-2-硫代-1H-环戊酮嘧啶-1-乙酸(20.0 g,1.0当量)和4-氟氯苄(13.4 g,1.05当量)为原料,制备2-(2-((4-氟苄基)硫基)-4-氧代-4,5,6,7-四氢-1H-环戊二烯并[d]嘧啶-1-基)乙酸的一般步骤如下:将2,3,4,5,6,7-六氢-4-氧-2-硫代-1H-环戊酮嘧啶-1-乙酸在水(112 mL)和异丙醇(20 mL)的混合物中浆化。加入50.9%的NaOH水溶液(13.82 g,1.99当量),随后用水(10 mL)洗涤,得到溶液。接着加入Na2CO3(1.50 g,0.16当量)并将溶液加热至40 ± 3°C。然后加入4-氟苄基氯,随后用异丙醇(12 mL)洗涤。反应混合物在40 ± 3°C下搅拌直至反应完成(约2.5小时)。冷却至20 ± 3°C后,加入甲酸(2.4 g,0.6当量),在30分钟内结晶产物。1小时内再加入第二批甲酸(6.9 g,1.7当量),浆液在20 ± 3°C下搅拌至少1小时。过滤浆液以分离产物,先后用水(32 mL)和异丙醇(8 mL)的混合物洗涤两次,再用异丙醇(40 mL)洗涤,最后在50°C下真空干燥,得到标题化合物,为灰白色固体(28.6 g,97%收率)。1H NMR (d6-DMSO) δ: 1.95 (2H, m), 2.57 (2H, t), 2.85 (2H, t), 4.4 (2H, s), 4.7 (2H, s), 7.15 (2H, dd), 7.45 (2H, dd), ~13.6 (1H, vbrs)。
参考文献:
[1] Patent: WO2011/146494, 2011, A1. Location in patent: Page/Page column 11
[2] Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 3, p. 839 - 843
[3] Bioorganic and Medicinal Chemistry Letters, 2018, vol. 28, # 4, p. 787 - 792