35808-68-5
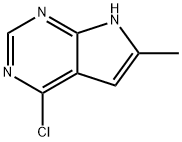 35808-68-5 结构式
35808-68-5 结构式
基本信息
4-Chloro-6-Methyl-7H-pyrr...
4-chloro-6-methyl-1H-pyrrolo[2,3-d]pyrimidine
4-Chloro-6-methyl-7H-pyrrolo[2,3-d]pyrimidine
7H-Pyrrolo[2,3-d]pyrimidine, 4-chloro-6-methyl-
1H-Pyrrolo[2,3-d]pyrimidine, 4-chloro-6-methyl-
物理化学性质
制备方法
![7-苯磺酰基-4-氯-6-甲基-7H-吡咯并[2,3-D]嘧啶](/CAS/GIF/252723-16-3.gif)
252723-16-3
![4-氯-6-甲基-7H-吡咯并[2,3-D]嘧啶](/CAS/GIF/35808-68-5.gif)
35808-68-5
化合物18.3的合成。将叔丁醇钾(450 mg,4 mmol)和7-苯磺酰基-4-氯-6-甲基-7H-吡咯并[2,3-d]嘧啶(250 mg,0.8 mmol)在四氢呋喃(7.5 mL)中的混合物于室温下搅拌16小时。反应完成后,向反应混合物中加入饱和碳酸氢钠溶液。用乙酸乙酯(3 × 100 mL)萃取反应混合物,合并有机层,依次用水和饱和盐水洗涤,无水硫酸钠干燥,减压浓缩。通过快速柱色谱法纯化残余物,以石油醚/乙酸乙酯(100/0至0/100,v/v)为洗脱剂,得到4-氯-6-甲基-7H-吡咯并[2,3-d]嘧啶(化合物18.3,118 mg,收率87%)。1H NMR (200 MHz, DMSO-d6) δ 12.5 (br s, 1H), 8.47 (s, 1H), 6.29 (s, 1H), 2.42 (s, 3H)。质谱(MS)m/z 168 [M + H]+。
参考文献:
[1] Patent: US2009/5359, 2009, A1. Location in patent: Page/Page column 26
[2] Beilstein Journal of Organic Chemistry, 2016, vol. 12, p. 1103 - 1110
[3] Patent: WO2012/158795, 2012, A1. Location in patent: Page/Page column 102
[4] Patent: WO2012/158764, 2012, A1. Location in patent: Page/Page column 215-216
[5] Patent: WO2013/191965, 2013, A1. Location in patent: Page/Page column 191
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW023580868504 | 4-氯-6-甲基-7H-吡咯并[2,3-d]嘧啶 4-Chloro-6-methyl-7H-pyrrolo[2,3-d]pyrimidine | 35808-68-5 | 1g | 732元 |
| 2025/12/22 | XW023580868503 | 4-氯-6-甲基-7H-吡咯并[2,3-d]嘧啶 4-Chloro-6-methyl-7H-pyrrolo[2,3-d]pyrimidine | 35808-68-5 | 250mg | 252元 |
| 2025/12/22 | XW023580868502 | 4-氯-6-甲基-7H-吡咯并[2,3-d]嘧啶 4-Chloro-6-methyl-7H-pyrrolo[2,3-d]pyrimidine | 35808-68-5 | 100mg | 108元 |
