37729-18-3
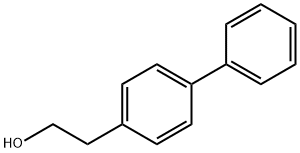 37729-18-3 结构式
37729-18-3 结构式
基本信息
4-羟乙基联苯
2-(4-联苯基)乙醇
2-([1,1'-联苯]-4-基)乙醇
2-([1,1-二联苯]-4-基)乙醇
2-([1,1'-联苯]-4-基)乙醇
[1,1biphenyl]-4-ethanol
2-(4-Biphenylyl)ethanol
2-(biphenyl-4-yl)ethanol
2-([1,1'-Biphenyl]-4-yl)
2-(4-phenylphenyl)ethanol
4-(2-Hydroxyethyl)biphenyl
2-([1,1'-Biphenyl]-4-yl)ethanol
2-([1,1'-Biphenyl]-4-yl)ethanol
2-[1,1'-Biphenyl]-4-yl-1-ethanol
物理化学性质
制备方法
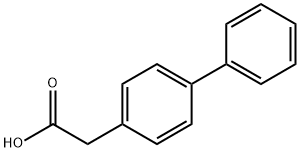
5728-52-9

37729-18-3
方法2:在氮气保护下,向装有四氢呋喃(THF)(40mL)的烧瓶中加入硼氢化钠(1.2g,31.7mmol),启动搅拌。随后,在20分钟内缓慢加入4-联苯基乙酸(5.0g,23.6mmol),保持反应混合物温度在20-30℃。加入THF冲洗液(5mL)后,继续搅拌20分钟。接着,在约1小时内缓慢滴加碘(2.9g,11.4mmol)的THF(10mL)溶液,同时维持反应温度在20-30℃。反应混合物在该温度下继续搅拌约1小时,直至薄层色谱(TLC)分析确认4-联苯乙酸完全转化。反应淬灭通过加入5wt%氢氧化钠水溶液(26mL)实现。充分混合后,静置分液,弃去下层水相。水相用乙酸异丙酯(2×25mL)萃取,合并有机相,用去离子水(4×20mL)洗涤。减压蒸发有机相,得到2-(联苯-4'-基)乙醇,为浅黄色结晶固体(4.6g,收率98.5%)。产物亦可参照方法1所述,通过乙酸异丙酯与庚烷的溶剂交换进行结晶纯化。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2005, vol. 13, # 15, p. 4740 - 4749
[2] Patent: WO2008/48609, 2008, A1. Location in patent: Page/Page column 86
[3] Patent: WO2008/5338, 2008, A1. Location in patent: Page/Page column 101
[4] Patent: WO2008/48609, 2008, A1. Location in patent: Page/Page column 55
[5] Patent: WO2014/144836, 2014, A2. Location in patent: Paragraph 0519; 0520