380151-86-0
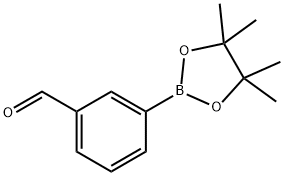 380151-86-0 结构式
380151-86-0 结构式
基本信息
3-甲酰苯硼酸频呢醇酯
3-醛基苯硼酸匹那醇酯
3-甲酰基苯硼酸频哪醇酯
3-甲酰基苯硼酸频哪醇酯(含有数量不等的酸酐)
3-(4,4,5,5-四甲基-1,3,2-二氧硼烷)苯甲醛
3-(4,4,5,5-四甲基-1,3,2-二氧硼戊烷-2-基)苯甲醛
3-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)苯甲醛
2-dioxaborolan-2-yl)-benzaldehyde
3-FORMYLPHENYLBORONIC ACID, PINACOL ESTER
3-Formylbenzeneboronic acid, pinacol ester
3-Formylbenzeneboronic acid, pinacol ester 95+%
3-FormylPhenylboronic acid neopentyl ester pinacol ester
2-(3-Formylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
3-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-BENZALDEHYDE
Benzaldehyde, 3-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-
物理化学性质
安全数据
制备方法
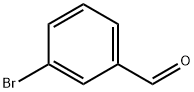
3132-99-8

73183-34-3

380151-86-0
向500 mL密封管中加入间溴苯甲醛(9.25 g,50.0 mmol)、乙酸钾(9.8 g,100 mmol)、双(频哪醇合)二硼烷(15.3 g,60.3 mmol)及1,4-二恶烷(50 mL)。用氮气对反应体系脱气15分钟。随后,加入Pd(dppf)Cl2(0.82 g,1.0 mmol),将反应混合物在90°C下搅拌反应过夜。反应完成后,将混合物通过硅藻土垫过滤,滤液用水和乙酸乙酯进行萃取分离。合并有机层,用无水Na2SO4干燥,减压浓缩。粗产物通过柱层析纯化,得到3-(4,4,5,5-四甲基-1,3,2-二氧硼戊烷-2-基)苯甲醛(11.5 g,收率99%),为无色液体。LCMS (M + H)+: 233.2; 1H NMR (DMSO-d6, 300 MHz) δ: 10.06 (s, 1H), 8.21 (s, 1H), 8.01-8.04 (d, 1H), 7.96-7.99 (d, 1H), 7.61-7.66 (t, 1H), 1.33 (s, 12H)。
参考文献:
[1] Patent: WO2014/202580, 2014, A1. Location in patent: Page/Page column 80; 81
[2] Patent: WO2014/202528, 2014, A1. Location in patent: Page/Page column 67
[3] Advanced Synthesis and Catalysis, 2016, vol. 358, # 6, p. 977 - 983
[4] Journal of Photochemistry and Photobiology A: Chemistry, 2018, vol. 364, p. 6 - 15
[5] Journal of the American Chemical Society, 2016, vol. 138, # 1, p. 84 - 87
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | T3709 | 3-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)苯甲醛 3-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzaldehyde | 380151-86-0 | 5g | 140元 |
| 2025/12/22 | XW0238015186005 | 3-(4,4,5,5-四甲基-1,3,2-二氧硼戊烷-2-基)苯甲醛 | 380151-86-0 | 100G | 790元 |
| 2025/12/22 | XW0238015186004 | 3-(4,4,5,5-四甲基-1,3,2-二氧硼戊烷-2-基)苯甲醛 | 380151-86-0 | 25G | 200元 |
