3846-73-9
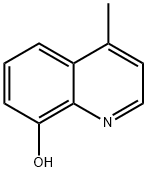 3846-73-9 结构式
3846-73-9 结构式
基本信息
4-甲基-8-羟基喹啉
4-Methylquinolin-8-o
4-Methylquinolin-8-ol
4-Methyl-8-quinolinol
8-Quinolinol, 4-Methyl-
4-METHYL-8-HYDROXYQUINOLINE
8-hydroxy-4-methylquinoline
物理化学性质
制备方法
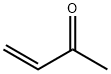
78-94-4
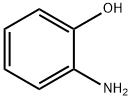
95-55-6

3846-73-9
将37%盐酸(45.5 mL)和邻氨基苯酚(10.91 g,0.1 mol)加入250 mL二颈圆底烧瓶中,充分搅拌并加热至120℃。缓慢滴加甲基乙烯基酮(14.32 mL,0.175 mol),保持回流反应6小时。反应完成后,冷却至室温,用氢氧化钠水溶液中和反应混合物。随后,用二氯甲烷(50 mL × 3)进行萃取。合并有机相,用无水硫酸镁干燥,减压浓缩除去溶剂。粗产物通过硅胶柱色谱纯化,以二氯甲烷为洗脱剂。最后,用二氯甲烷与正己烷(1:1,v/v)混合溶剂重结晶,得到无色固体4-甲基-8-羟基喹啉(4.38 g,产率27.5%)。核磁共振氢谱(400 MHz, (CD3)2CO, Me4Si)数据如下:δ 8.68(d, 1H, J = 4.4 Hz),7.55(dd, 1H, J1 = 8.4 Hz, J2 = 1.5 Hz),7.51(dd, 1H, J1 = 8.4 Hz, J2 = 7.2 Hz),7.41(dd, 1H, J1 = 4.4 Hz, J2 = 0.8 Hz),7.12(dd, 1H, J1 = 7.2 Hz, J2 = 1.5 Hz),2.71(d, 3H, J = 0.8 Hz)。
参考文献:
[1] Chemical Communications, 2015, vol. 51, # 84, p. 15458 - 15461
[2] Journal of the Chinese Chemical Society, 2004, vol. 51, # 4, p. 735 - 742
[3] Tetrahedron Letters, 2016, vol. 57, # 25, p. 2797 - 2799
[4] Journal of the American Chemical Society, 1949, vol. 71, p. 3986
[5] Patent: US4843082, 1989, A
