38923-08-9
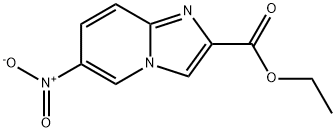 38923-08-9 结构式
38923-08-9 结构式
基本信息
6-硝基咪唑[1,2-A]吡啶-2-羧酸乙酯
6-硝基咪唑并[1,2-A]吡啶-2-羧酸乙酯
6-硝基咪唑[1,2-ALPHA]吡啶-2-羧酸乙酯
ethyl 6-nitroH-imidazo[1,2-a]pyridine-2-carboxylate
6-NITROIMIDAZO[1,2-A]PYRIDINE-2-CARBOXYLIC ACID ETHYL ESTER
6-nitroimidazo[1,2-α]pyridine-2-carboxylic acid ethyl ester
Imidazo[1,2-a]pyridine-2-carboxylic acid, 6-nitro-, ethyl ester
6-NITROIMIDAZO[1,2-A]PYRIDINE-2-CARBOXYLIC ACID ETHYL ESTER, 95+%
物理化学性质
制备方法
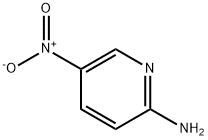
4214-76-0
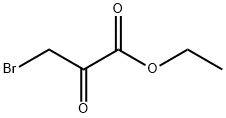
70-23-5
![6-硝基咪唑[1,2-A]吡啶-2-羧酸乙酯](/CAS/GIF/38923-08-9.gif)
38923-08-9
以2-氨基-5-硝基吡啶(1.5 g,10.80 mmol)和3-溴丙酮酸乙酯(1.6 mL,13.00 mmol)为原料,在乙醇(15.0 mL)中溶解后,回流反应16小时。反应完成后,过滤反应混合物,收集浅黄色固体。滤液用乙酸乙酯萃取,依次用饱和碳酸氢钠水溶液和盐水洗涤。有机层经无水硫酸钠干燥后,减压浓缩。残余物通过快速柱色谱(硅胶,正己烷:乙酸乙酯 = 2:1)纯化,得到6-硝基咪唑[1,2-A]吡啶-2-羧酸乙酯(2.3 g,收率90%)为黄色固体。LC/MS ESI(+):m/z 236 [M + H]+。1H NMR (300 MHz, CDCl3) δ: 9.30 (s, 1H), 8.38 (s, 1H), 8.06 (d, 1H, J = 10.0 Hz), 7.84 (d, 1H, J = 10.4 Hz), 4.50 (q, 2H, J = 7.2 Hz), 1.46 (t, 3H, J = 7.2 Hz)。
参考文献:
[1] Patent: WO2014/196793, 2014, A1. Location in patent: Page/Page column 64; 65
[2] Molecular Pharmaceutics, 2015, vol. 12, # 6, p. 1813 - 1835
[3] Patent: WO2015/185142, 2015, A1. Location in patent: Page/Page column 20
[4] Patent: WO2008/100423, 2008, A1. Location in patent: Page/Page column 111
[5] Patent: EP1657242, 2006, A1. Location in patent: Page/Page column 27
