400777-06-2
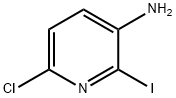 400777-06-2 结构式
400777-06-2 结构式
基本信息
2-氯-3-氨基-6-碘吡啶
3-氨基-6-氯-2-碘吡啶
3-氨基-2-氯-6-碘吡啶
2-碘-3-胺基-6-氯吡啶
2-碘-3-氨基-6-氯吡啶
3-Amino-6-chloro-2-iodopyridine
3-Amino-2-chloro-6-iodopyridine
2-Iodo-6-chloro-pyridin-3-amine
3-Pyridinamine, 6-chloro-2-iodo-
6-chloro-2-iodo-pyridin-3-ylamine
3-Amino-2-chloro-6-iodopyridine ISO 9001:2015 REACH
物理化学性质
制备方法
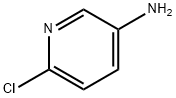
5350-93-6

400777-06-2
步骤a:6-氯-2-碘吡啶-3-胺的合成 向6-氯吡啶-3-胺(10.0g,77.8mmol)的乙醇(150mL)溶液中依次加入硫酸银(12.1g,38.9mmol)和碘(23.7g,93.4mmol)。将反应混合物在20℃下搅拌过夜。反应完成后,通过减压蒸馏除去溶剂。向残余物中加入水(100mL)和乙酸乙酯(200mL),分离有机层。水层用乙酸乙酯(100mL×3)萃取三次。合并有机层,用无水硫酸钠干燥,减压浓缩得到粗产物。粗产物通过硅胶柱色谱纯化(洗脱剂:石油醚/乙酸乙酯=7:1),得到6-氯-2-碘吡啶-3-胺(17.1g,收率86%)。 1H NMR (DMSO, 300MHz) δ 7.16 (d, J = 8.4Hz, 1H), 7.01 (d, J = 8.4Hz, 1H), 5.57 (s, 2H)。
参考文献:
[1] Patent: US2009/253736, 2009, A1. Location in patent: Page/Page column 23
[2] Patent: EP2826781, 2015, A1. Location in patent: Paragraph 0077-0078
[3] Patent: KR2015/9461, 2015, A. Location in patent: Paragraph 0195; 0196
[4] Patent: WO2013/10880, 2013, A1. Location in patent: Page/Page column 82
[5] Patent: EP2548876, 2013, A1. Location in patent: Paragraph 0275-0278
