40107-93-5
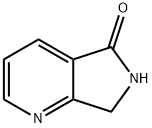 40107-93-5 结构式
40107-93-5 结构式
基本信息
6,7-二氢吡咯[3,4-B]并吡啶-5-酮
6,7-二氢-5H-吡咯并[3,4-B]吡啶-5-酮
2-(Aminomethyl)nicotinic acid lactam
5H,6H,7H-Pyrrolo[3,4-b]pyridin-5-one
5,7-Dihydropyrrolo[3,4-b]pyridin-5-one
6,7-dihydropyrrolo[3,4-b]pyridin-5-one
6,7-Dihydro-5H-pyrrolo[3,4-b]pyridin-5-one
6,7-Dihydro-5-oxo-5H-pyrrolo[3,4-b]pyridine
5H-Pyrrolo[3,4-b]pyridin-5-one, 6,7-dihydro-
6,7-dihydropyrrolo[3,4-b]pyridin-5-one ISO 9001:2015 REACH
物理化学性质
制备方法
![7-羟基-6,7-二氢-5H-吡咯并[3,4-B]吡啶-5-酮](/CAS/GIF/115012-09-4.gif)
115012-09-4
![6,7-二氢吡咯[3,4-B]并吡啶-5-酮](/CAS2/GIF/40107-93-5.gif)
40107-93-5
以7-羟基-6,7-二氢-5H-吡咯并[3,4-b]吡啶-5-酮为原料合成6,7-二氢-5H-吡咯并[3,4-b]吡啶-5-酮的一般步骤:向7-羟基-6,7-二氢-5H-吡咯并[3,4-b]吡啶-5-酮(0.32 g,2.13 mmol,1.0 equiv)的乙酸(8.6 mL)溶液中加入锌粉(0.56 g,8.50 mmol,4.0 equiv)。将悬浮液加热回流24小时。反应完成后,将混合物冷却至室温,并通过硅藻土垫过滤。滤液经减压浓缩,残余物用二氯甲烷(CH2Cl2)溶解。向该溶液中加入无水氯化钙(CaCl2),过滤除去不溶物。滤液再次减压浓缩,残余物通过柱色谱法纯化,先使用乙酸乙酯(EtOAc)作为洗脱剂,随后改用乙酸乙酯/甲醇(95:5, v/v)混合溶剂作为洗脱剂。收集目标馏分,浓缩后用乙醇(EtOH)重结晶,得到6,7-二氢-5H-吡咯并[3,4-b]吡啶-5-酮(0.11 g,收率38%)为白色固体。熔点:206°C(分解)。1H NMR (300 MHz, DMSO-d6) δ 8.66 (br s, 1H), 8.63 (d, J = 4.9 Hz, 1H), 7.95 (d, J = 7.05 Hz, 1H), 7.38 (dd, J = 7.6/4.9 Hz, 1H), 4.3 (s, 2H); 13C NMR (75 MHz, DMSO-d6) δ 168.7, 164.9, 153.0, 131.9, 126.4, 123.6, 47.1; GC-MS m/z 134 (M)+; IR (KBr) 3352, 3199, 1707, 1607, 1411, 1138 cm?1。元素分析计算值(C7H6N2O):C, 62.68; H, 4.51; N, 20.88。实测值:C, 62.73; H, 4.50; N, 20.50。
参考文献:
[1] Bulletin of the Chemical Society of Japan, 1987, vol. 60, # 11, p. 4178 - 4180
[2] European Journal of Medicinal Chemistry, 2012, vol. 55, p. 58 - 66,9
[3] Patent: CN105541833, 2016, A. Location in patent: Paragraph 0155; 0156; 0157
