406235-30-1
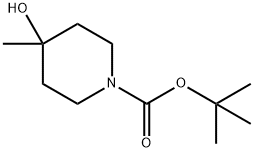 406235-30-1 结构式
406235-30-1 结构式
基本信息
1-BOC-4-羟基-4-甲基哌啶
4-羟基-4-甲基哌啶-1-羧酸叔丁酯
1-BOC-4-METHYL-PIPERIDIN-4-OL
1-boc-4-methyl-piperidine-4-ol
N-Boc-4-Hydroxy-4-methylpiperidine
1-Boc-4-hydroxy-4-methylpiperidine
N-BOC-4-METHYL-4-HYDROXY PIPERIDINE
4-HYDROXY-4-METHYL-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
1-Piperidinecarboxylicacid,4-hydroxy-4-Methyl-,1,1-diMethylethylester
4-Hydroxy-4-methylpiperidine-1-carboxylic acid tert-butyl estercarboxylate
物理化学性质
制备方法
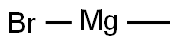
75-16-1
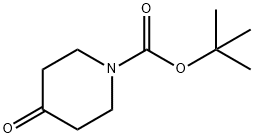
79099-07-3

406235-30-1
a) 将N-叔丁氧羰基-4-哌啶酮(1.0g,5.0mmol)溶解于无水乙醚(10mL)中,并在氮气保护下冷却至-10℃。缓慢滴加甲基溴化镁的乙醚溶液(3M,2.5mL,7.5mmol),滴加完毕后将反应混合物搅拌5分钟。移除冷却浴,使反应混合物升温至室温并继续搅拌2小时。反应完成后,用饱和氯化铵水溶液(10mL)淬灭反应,随后加入水(20mL)和乙醚(20mL)进行稀释。分离水相,用乙醚(2×30mL)萃取,合并有机相后用盐水(50mL)洗涤,无水硫酸钠干燥,减压浓缩,得到N-BOC-4-甲基-4-羟基哌啶,为无色油状物(1.05g,收率97%)。1H NMR(400MHz,CDCl3)δ3.76-3.64(m,2H),3.29-3.16(m,2H),1.66(s,1H),1.45(s,9H),1.25(s,3H),溶剂峰未明确归属。
参考文献:
[1] Journal of the American Chemical Society, 2013, vol. 135, # 41, p. 15342 - 15345
[2] Patent: WO2014/128465, 2014, A1. Location in patent: Page/Page column 156
[3] Patent: WO2009/151598, 2009, A1. Location in patent: Page/Page column 148-149
[4] Patent: WO2014/164409, 2014, A1. Location in patent: Page/Page column 32; 33
[5] Patent: WO2014/164428, 2014, A1. Location in patent: Page/Page column 25
