426844-76-0
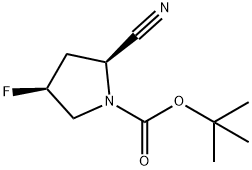 426844-76-0 结构式
426844-76-0 结构式
基本信息
(2S,4S)-1-BOC-2-氰基-4-氟吡咯烷
1-叔丁氧羰基-(2S,4S)-2-氰基-4-氟吡咯烷
(2S,4S)-2-氰基-4-氟吡咯烷-1-甲酸叔丁酯
(2S,4S)-2-氰基-4-氟吡咯烷-1-羧酸叔丁酯
N-Boc-cis-4-fluoro-L-prolinonitrile
N-t-Boc-cis-4-Fluoro-L-prolinonitrile
(2S,4S)-1-Boc-2-cyano-4-fluoropyrrolidine
1-Boc-(2S,4S)-2-cyano-4-fluoropyrrolidine
-tert-Butyl 2-cyano-4-fluoropyrrolidine-1-carboxylate
(2S,4S)-1-tert-Butoxycarbonyl-2-cyano-4-fluoropyrrolidine
(2S,4S)-tert-Butyl 2-cyano-4-fluoropyrrolidine-1-carboxylate
(2S,4S)-2-cyano-4-fluoropyrrolidine-1- tert-butyl carboxylate
1-Pyrrolidinecarboxylic acid, 2-cyano-4-fluoro-, 1,1-dimethylethyl ester, (2S,4S)-
物理化学性质
制备方法
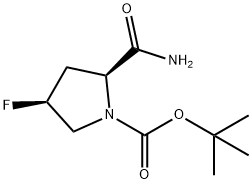
426844-22-6

426844-76-0
第2步: (2S,4S)-1-Boc-2-氰基-4-氟吡咯烷的合成 向搅拌并冷却至0℃的(2S,4S)-N-Boc-4-氟吡咯烷-2-甲酰胺(10g, 43.10mmol)的无水THF(50ml)溶液中依次加入三乙胺(13.93g, 138mmol)和三氟乙酸酐(14.5g, 69.05mmol)。将反应混合物在0℃下继续搅拌1小时。反应完成后,用100ml水淬灭反应,并用氯仿(2×100ml)萃取。合并有机相,依次用水(2×100ml)和盐水(50ml)洗涤,无水硫酸钠干燥。减压浓缩溶剂,得到灰白色固体产物9.0g,收率97.6%。 IR(KBr, cm-1): 2979, 2243, 1387, 1240, 1168, 1123, 1072, 960。 1H NMR(CDCl3, 300MHz) δ: 1.49-1.53(d, 旋转异构体, 9H), 2.25-2.47(m, 1H), 2.64(t, J=14.7Hz, 1H), 3.52(dd, J=9.6,3.6Hz, 0.5H, 旋转异构体), 3.64(dd, J=9.3,3.3Hz, 0.5H, 旋转异构体), 3.73-3.94(m, 1H), 4.64(d, J=8.7Hz, 0.6H, 旋转异构体), 4.76(d, J=8.7Hz, 0.4H, 旋转异构体), 5.31(brd, J=51.3Hz, 1H)。
参考文献:
[1] Chemical and Pharmaceutical Bulletin, 2008, vol. 56, # 8, p. 1110 - 1117
[2] Patent: WO2005/75426, 2005, A1. Location in patent: Page/Page column 35-36
[3] Patent: WO2006/90244, 2006, A1. Location in patent: Page/Page column 29
[4] Patent: WO2006/40625, 2006, A1. Location in patent: Page/Page column 44
[5] Patent: WO2007/99385, 2007, A1. Location in patent: Page/Page column 26