42923-79-5
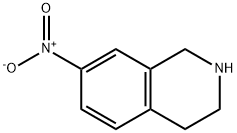 42923-79-5 结构式
42923-79-5 结构式
基本信息
1,2,3,4-tetrahydro-7-nitroisoquinoline
7-NITRO-1,2,3,4-TETRAHYDRO-ISOQUINOLINE
Isoquinoline, 1,2,3,4-tetrahydro-7-nitro-
1,2,3,4-Tetrahydro-7-nitroisoquinoline 98%
7-Nitro-1,2,3,4-tetrahydro-isoquinoline ,97%
物理化学性质
制备方法
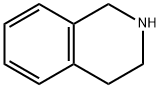
91-21-4

42923-79-5
在研钵中,将2g P2O5/硅胶(65%w/w)与1,2,3,4-四氢异喹啉(10mmol,1.33g)混合研磨30秒,随后缓慢滴加5ml 65% HNO3,继续在室温下研磨20分钟,直至混合物呈现深黄色。通过TLC(正己烷:EtOAc 70:30)监测反应进程,确认1,2,3,4-四氢异喹啉完全消失(约30分钟)。反应完成后,向混合物中加入乙醚(50ml),通过短硅胶垫过滤分离固体,并用乙醚(2×15ml)洗涤。合并的有机相用10% NaHCO3溶液(20ml)洗涤,随后用MgSO4干燥。减压浓缩除去溶剂,残余物通过柱色谱(正己烷:EtOAc,2:1)纯化,得到7-硝基-1,2,3,4-四氢异喹啉(2b)(8mmol,1.4g,收率80%),为黄色固体,熔点121℃。1H NMR (δ): 8.05 (m, 2H), 7.60 (m, 1H), 3.82 (s, 2H), 3.38 (t, 2H, J = 7.4Hz), 3.12 (t, 2H, J = 7.4Hz), 2.83 (s, 1H)。13C NMR (δ): 150.6, 145.0, 140.3, 129.6, 122.6, 121.4, 46.9, 44.1, 28.1。高分辨质谱(EMS) [M+H]+ C9H10N2O2,计算值179.0740,实测值179.1121。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2011, vol. 19, # 24, p. 7435 - 7440
[2] Journal of Heterocyclic Chemistry, 1985, vol. 22, p. 329 - 331
[3] Journal of Medicinal Chemistry, 2003, vol. 46, # 5, p. 831 - 837
[4] Patent: WO2004/60902, 2004, A2. Location in patent: Page/Page column 15
[5] Patent: US9138427, 2015, B2. Location in patent: Page/Page column 307

