4295-99-2
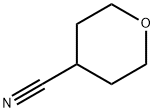 4295-99-2 结构式
4295-99-2 结构式
基本信息
4-氰基四氢吡喃
四氢吡喃-4-氰基
四氢-2H-吡喃-4-腈
4-氰基四氢-4H-吡喃
四氢-2H-吡喃-4-甲腈
4-氰基四氢吡喃(CAS号:4295-99-2)
4-cyanotetrahydropiran
4-Cyanotetrahydropyran
4-CYANOTETRAHYDRO-4H-PYRAN
Tetrahydro-pyran-4-carbonitrile
2H-Pyran-4-carbonitrile, tetrahydro-
4-Cyanotetrahydro-4H-pyran manufacturer
4-Cyanotetrahydro-4H-pyran ISO 9001:2015 REACH
tetrahydro-2H-pyran-4-carbonitrile(SALTDATA: FREE)
物理化学性质
制备方法
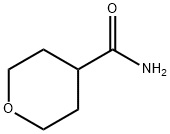
344329-76-6

4295-99-2
以四氢吡喃-4-甲酰胺为原料合成4-氰基四氢吡喃的一般步骤如下:将亚硫酰氯(10.0 mL,137 mmol)缓慢加入四氢吡喃-4-甲酰胺(3.0 g,23 mmol)中,反应混合物在回流条件下搅拌4小时。反应完成后,将混合物小心倾倒在冰上,并用50%氢氧化钠溶液调节pH至14。随后,用乙酸乙酯(3×50 mL)萃取水相,合并有机相并用无水硫酸钠干燥。减压浓缩有机相,得到浅黄色油状产物(2.4 g,收率94%),无需进一步纯化。产物的结构通过以下表征数据确认:IR (νmax, cm-1): 2961, 2932, 2851, 2240, 1468, 1446, 1390, 1242, 1125, 1066, 1011; 1H NMR (500 MHz, CDCl3): δ 3.91 (2H, ddd, J = 11.9, 6.3, 3.6 Hz, 2×2-HA), 3.61 (2H, ddd, J = 11.9, 7.8, 3.3 Hz, 2×2-HB), 2.91-2.85 (1H, m, 4-H), 1.99-1.92 (2H, m, 2×3-HA), 1.91-1.84 (2H, m, 2×3-HB); 13C NMR (75 MHz, CDCl3): δ 121.2, 65.6, 28.9, 25.3; HRMS (ESI+): 计算值C6H10NO ([M+H]+): 112.0756, 实测值: 112.0754, Δ -1.8 ppm。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2015, vol. 23, # 11, p. 2629 - 2635
[2] Patent: US2007/111984, 2007, A1. Location in patent: Page/Page column 45
[3] Journal of the American Chemical Society, 1943, vol. 65, p. 370
[4] Patent: US2009/253679, 2009, A1. Location in patent: Page/Page column 54-55
