443912-70-7
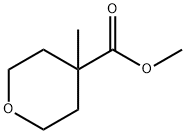 443912-70-7 结构式
443912-70-7 结构式
基本信息
甲基 4-甲基噁烷-4-甲酸基酯
4-甲基-四氢-2H-吡喃-4-羧酸甲酯
Methyl 4-Methyloxane-4-carboxylate
4-methyl-4-oxanecarboxylic acid methyl ester
Methyl 4-Methyltetrahydro-2H-pyran-4-carboxylate
methyl tetrahydro-4-methyl-2H-pyran-4-carboxylate
4-Methyltetrahydropyran-4-carboxylic acid methyl ester
Tetrahydro-4-methyl-2H-pyran-4-carboxylic acid methyl ester
2H-Pyran-4-carboxylicacid, tetrahydro-4-methyl-, methyl este...
2H-Pyran-4-carboxylicacid,tetrahydro-4-methyl-,methylester(9CI)
制备方法
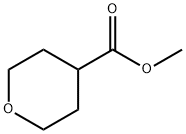
110238-91-0

74-88-4

443912-70-7
在-78℃下,将22.1 mL正丁基锂(35.4 mmol,1.6 M己烷溶液)缓慢滴加到4.91 mL(35.0 mmol)二异丙胺在45 mL四氢呋喃中的溶液中,混合物在-78℃下搅拌10分钟,随后在0℃下搅拌25分钟。然后,在-78℃下加入5.00 g(34.7 mmol)四氢-2H-吡喃-4-羧酸甲酯在45 mL四氢呋喃中的溶液,混合物在-78℃下继续搅拌15分钟。接着,在-78℃下滴加2.16 mL(34.7 mmol)碘甲烷,反应混合物缓慢升温至-25℃,并在室温下搅拌过夜。反应完成后,加入80 mL 0.1 N盐酸终止反应,分离有机相和水相。水相用乙酸乙酯萃取两次,合并有机相,用硫酸镁干燥,过滤后减压浓缩。将所得悬浮液与20 mL甲基叔丁基醚一起研磨,抽滤出沉淀物,减压浓缩母液,得到4-甲基-四氢-2H-吡喃-4-羧酸甲酯。产量:5.88 g(纯度95%,定量收率)。1H NMR(400 MHz,DMSO-d6)δ(ppm):3.70-3.64(m,2H),3.64(s,3H),3.37-3.30(m,2H),1.94-1.88(m,2H),1.45-1.37(m,2H),1.16(s,3H)。
参考文献:
[1] Patent: US2016/52884, 2016, A1. Location in patent: Paragraph 1748-1750
[2] Patent: KR2015/137095, 2015, A. Location in patent: Paragraph 2557-2560
[3] Patent: US2018/148451, 2018, A1. Location in patent: Paragraph 0141; 0142; 0143
[4] Journal of Medicinal Chemistry, 2002, vol. 45, # 14, p. 2994 - 3008
[5] Patent: WO2005/58881, 2005, A1. Location in patent: Page/Page column 29-30
