457889-46-2
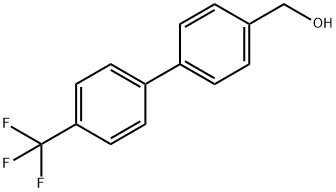 457889-46-2 结构式
457889-46-2 结构式
基本信息
4'-(三氟甲基)联苯-4-甲醇
RARECHEM AL BD 1218
4-(4-Trifluoromethyl)Benzyl Alcohol
4’-(Trifluoromethyl)biphenyl-4-methanol
(4'-TRIFLUOROMETHYLBIPHENYL-4-YL)-METHANOL
1-(4^-Trifluoromethylbiphenyl-4-yl)methanol
4-(4-(Trifluoromethyl)phenyl)benzyl alcohol
[4-[4-(trifluoromethyl)phenyl]phenyl]methanol
4'-(TrifluoroMethyl)-[1,1'-biphenyl]-4-Methanol
(4-[4-(TRIFLUOROMETHYL)PHENYL]PHENYL)METHAN-1-OL
制备方法
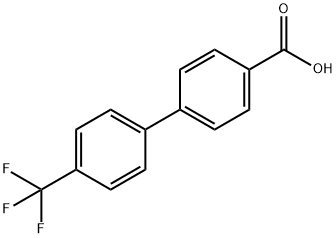
195457-71-7

457889-46-2
在氮气保护下,将LiAlH4(2.0M THF溶液,3.0mL,6.00mmol)溶于无水THF(10mL)中,冷却至0℃。向此搅拌中的混合物逐滴加入4'-[4-(三氟甲基)苯基]苯甲酸(0.4g,1.5mmol)的无水THF(10mL)溶液。反应混合物在室温下搅拌反应4小时。随后,在0℃下缓慢加入H2O(0.23mL)、3.0M KOH溶液(0.23mL)和H2O(0.77mL)。混合物在0℃下继续搅拌1小时,过滤去除固体残留物,有机相用Na2SO4干燥。再次过滤有机溶液,浓缩至干。使用Teledyne ISCO装置,通过柱色谱法纯化粗产物,洗脱剂为环己烷/乙酸乙酯(100:0至70:30),得到4'-三氟甲基-4-联苯甲醇(0.3g,79%),为白色固体。1H NMR(DMSO-d6)数据如下:δ4.56(d,J=5.7Hz,2H),5.25(t,J=5.7Hz,1H),7.45(d,J=8.1Hz,2H),7.70(d,J=8.1Hz,2H),7.81(d,J=8.1Hz,2H),7.89(d,J=8.1Hz,2H)。
参考文献:
[1] Patent: WO2013/78430, 2013, A1. Location in patent: Paragraph 0353
[2] Journal of Medicinal Chemistry, 2014, vol. 57, # 23, p. 10101 - 10111
[3] Patent: US9353075, 2016, B2. Location in patent: Page/Page column 122
[4] Patent: WO2009/71504, 2009, A1. Location in patent: Page/Page column 65
[5] Patent: WO2010/15653, 2010, A1. Location in patent: Page/Page column 39
