4766-33-0
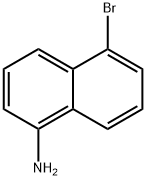 4766-33-0 结构式
4766-33-0 结构式
基本信息
5-溴萘-1-胺
1-氨基-5-溴萘
5-Bromo-1-naphthalenamine
5-bromonaphthalen-1-amine
1-NaphthalenaMine,5-broMo-
5-BROMO-NAPHTHALEN-1-YLAMINE
物理化学性质
安全数据
制备方法
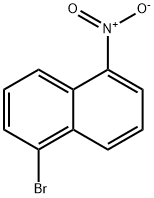
5328-76-7

4766-33-0
5-氨基-1-溴萘(B)的合成:将5-硝基-1-溴萘(A)(2.5 g,10 mmol)溶于EtOH/AcOH/二恶烷/H2O(2:2:2:1,30 mL)的混合溶剂中。向该溶液中加入铁粉(5.6 g,100 mmol)和2滴2N HCl。反应混合物在100℃下搅拌2小时。反应完成后,蒸发去除溶剂,将残余物溶解于100 mL二氯甲烷(DCM)中,用5% NaHCO3溶液(3×30 mL)洗涤,无水Na2SO4干燥。蒸发溶剂后,粗产物通过硅胶柱色谱纯化,使用DCM/己烷(1:1)作为洗脱剂,得到化合物B,为白色粉末(3.0 g,97%收率)。熔点65-66℃。1H NMR(400 MHz,CDCl3)δ 7.79(d,J = 8.8 Hz,1H),7.76(d,J = 8.0 Hz,1H),7.71(d,J = 8.4 Hz,1H),7.39(t,J = 7.6 Hz,1H),7.28(t,J = 7.2 Hz,1H),6.83(d,J = 7.2 Hz,1H),4.18(s,2H);13C NMR(100 MHz,CDCl3)δ 142.5,133.0,130.4,128.0,125.1,125.0,123.9,121.0,118.4,110.9;ESI-MS(m/z)计算值C10H9BrN [M+H]+ 222.0,实测值222.1。元素分析计算值(C10H8BrN):C,54.08;H,3.63;N,6.31。实测值:C,53.81;H,3.46;N,6.11。
参考文献:
[1] New Journal of Chemistry, 2005, vol. 29, # 4, p. 579 - 586
[2] Patent: US2007/274922, 2007, A1
[3] Journal of Organic Chemistry, 2017, vol. 82, # 7, p. 3873 - 3879
[4] Organic Letters, 2018, vol. 20, # 4, p. 1261 - 1264
[5] Patent: US2018/223102, 2018, A1. Location in patent: Paragraph 0260-0262
