494-08-6
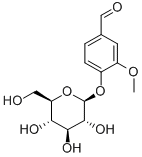 494-08-6 结构式
494-08-6 结构式
基本信息
4-(Β-D-葡萄糖基)-3-甲氧基苯甲醛
4-(Β-D-葡萄糖氧基)-3-甲氧基苯甲醛
4-(Β-D-葡萄糖氧基)-3-甲氧基苯甲醛
4-(BETA-D-葡萄糖基)-3-甲氧基苯甲醛
4-(BETA-D-葡萄糖基氧基)-3-甲氧基苯甲醛
4-(Β-D-GLUCOPYRANOSYLOXY)-3-METHOXYBENZALDEHYDE4-(Β-D-葡萄糖基)-3-甲氧基苯甲醛
4-(Β-D-葡萄糖氧基)-3-甲氧基苯甲醛,4-(Β-D-GLUCOPYRANOSYLOXY)-3-METHOXYBENZALDEHYDE
Vanilloside
GLUCOVANILLIN
Vanillin Glucoside
VANILLIN D-GLUCOSIDE
Glucovanillin - Synthetic
Vanillin 4-O-β-D-Glucoside
Vanillin 4-O-b-D-Glucoside
Vanillin β-D-Glucopyranoside
Vanillin 4-O-beta-D-glucoside
物理化学性质
制备方法
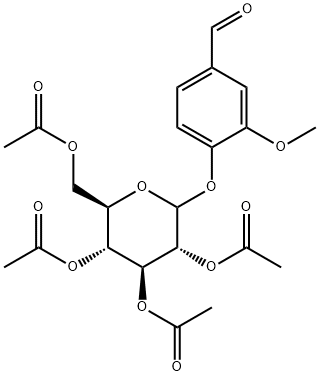
51482-43-0

494-08-6
以4-O-(2',3',4',6'-四氧-乙酰基-Β-D-吡喃葡萄糖基)香草醛为原料合成3-甲氧基-4-(((2S,3R,4S,5S,6R)-3,4,5-三羟基-6-(羟甲基)四氢-2H-吡喃-2-基)氧基)苯甲醛的一般步骤:将香草醛2,3,4,6-四-O-乙酰基-β-D-吡喃葡萄糖苷(1.36 g,3.0 mmol)溶解于THF/CH3OH/H2O(33 mL,体积比为5:5:1)的混合溶剂中,随后加入K2CO3(0.83 g,6.0 mmol)。反应混合物在40℃下搅拌20分钟后,用1M HCl溶液淬灭反应,随后进行浓缩。残余物通过硅胶柱色谱法(洗脱剂为CH2Cl2/CH3OH,体积比为15:1)进行纯化,得到目标产物4,为白色固体(0.88 g,产率94%)。产物的比旋光度为[a]D20 -11.4(c 1.0,H2O)。1H NMR(DMSO-d6,400 MHz)δ:9.86(s,1H,CHO),7.52(dd,J = 8.3, 1.5 Hz,1H,Ph-H),7.43(d,J = 1.4 Hz,1H,Ph-H),7.28(d,J = 8.4 Hz,1H,Ph-H),5.39(d,J = 4.4 Hz,1H,-OH),5.16(d,J = 3.7 Hz,1H,-OH),5.09(d,J = 5.2 Hz,2H,-OH,H-1),4.59(t,J = 5.6 Hz,1H,-OH),3.84(s,3H,-OMe),3.66(dd,J = 11.2, 4.7 Hz,1H,H-3),3.48-3.45(m,1H,H-2),3.40-3.37(m,1H,H-4),3.30-3.25(m,2H,H-6),3.20-3.14(m,1H,H-5)。13C NMR(DMSO-d6,150 MHz)δ:191.66(CHO),151.78,149.34,130.56,125.43,114.58,110.52,99.41(C-1),77.17,76.84,73.11,69.58,60.62,55.70。ESI-HRMS m/z [M+Na]+计算值C14H18NaO8为337.0894,实测值为337.0901。
参考文献:
[1] Carbohydrate Research, 2018, vol. 460, p. 41 - 46
[2] Journal of Agricultural and Food Chemistry, 2009, vol. 57, # 5, p. 2056 - 2064
[3] Synthesis (Germany), 2016, vol. 48, # 20, p. 3575 - 3588