494799-17-6
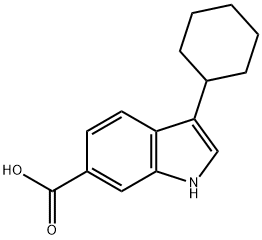 494799-17-6 结构式
494799-17-6 结构式
基本信息
3-环己烷吲哚-6-甲酸
3-环己基-6-吲哚羧酸
3-环己基-1H-吲哚-6-羧酸
3-Cyclohexyl-6-indolecarboxylic acid
3-Cyclohexane-1H-indole-6-carboxylic acid
1H-Indole-6-carboxylic acid, 3-cyclohexyl-
制备方法
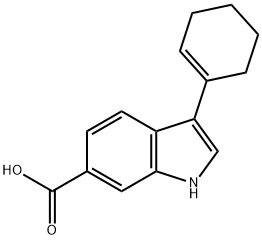
494799-16-5

494799-17-6
第2步: 甲基-3-环己基-1H-吲哚-6-羧酸酯的合成; 将3-环己烯基-1H-吲哚-6-羧酸(来自步骤1)溶于THF/MeOH混合溶剂(0.5M,1:1,v/v)中,加入Pd(OH)2/C催化剂(0.1当量,20%)。在60 psi氢气压力下氢化14小时。反应完成后,通过硅藻土垫过滤以除去催化剂。将滤液浓缩至干,得到3-环己基-1H-吲哚-6-羧酸,收率90%,为白色固体。1H NMR (300 MHz, DMSO-d6, 300 K) δ 1.20-1.53 (m, 5H), 1.70-1.87 (m, 3H), 1.90-2.02 (m, 2H), 2.69-2.86 (m, 1H), 7.40 (s, 1H), 7.55-7.65 (m, 2H), 8.0 (s, 1H), 11.40 (s, 1H); MS (ES+) m/z 244 (M + H)+。将上述产物溶于MeOH(0.4M)中,在0℃下滴加亚硫酰氯(0.5当量),然后回流24小时。反应完成后,减压蒸馏除去挥发物,得到甲基-3-环己基-1H-吲哚-6-羧酸酯,收率100%,为固体。
参考文献:
[1] Patent: WO2006/29912, 2006, A1. Location in patent: Page/Page column 21-22
[2] Patent: WO2009/147121, 2009, A1. Location in patent: Page/Page column 65-66
[3] Patent: WO2009/80836, 2009, A2. Location in patent: Page/Page column 56
[4] Patent: WO2006/76529, 2006, A1. Location in patent: Page/Page column 135
[5] Patent: US2007/78122, 2007, A1. Location in patent: Page/Page column 174
