501907-61-5
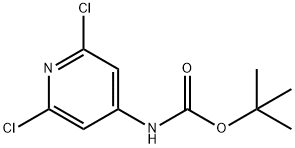 501907-61-5 结构式
501907-61-5 结构式
基本信息
N-BOC-4-氨基-2,6-二氯吡啶
(2,6-二氯吡啶-4-基)氨基甲酸叔丁酯
tert-Butyl (2,6-dichloropyridin-4-yl)
t-butyl 2,6-dichloropyridin-4-yl-carbamate
1-N-Boc-4-aMino-2-chloro-3-forMylpyridine
ert-Butyl(2,6-dichloropyridin-4-yl)carbamate
tert-butyl (2,6-dichloropyridin-4-yl)carbamate
tert-butyl N-(2,6-dichloropyridin-4-yl)carbamate
(2,6-Dichloropyridin-4-yl)carbamic acid tert-butyl ester
(2,6-dichloro-4-pyridinyl)-, 1,1-diMethylethyl ester (9CI)
Carbamic acid, N-(2,6-dichloro-4-pyridinyl)-, 1,1-dimethylethyl ester
物理化学性质
制备方法
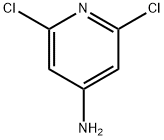
2587-02-2

24424-99-5

501907-61-5
步骤1. 在室温下,将4-氨基-2,6-二氯吡啶(5.04 g,30.3 mmol)溶解于二氯甲烷(DCM,100 mL)中,依次加入4-二甲氨基吡啶(DMAP,0.61 g,4.99 mmol)和二碳酸二叔丁酯(Boc2O,13.71 g,60.9 mmol)。将反应混合物在室温下搅拌过夜。反应完成后,依次用饱和氯化铵水溶液(3 × 30 mL)和盐水(40 mL)洗涤有机相,随后用无水硫酸钠干燥并浓缩。将残余物溶解于四氢呋喃(THF,30 mL)和水(30 mL)的混合溶剂中,加入氢氧化钠(4.5 g,112 mmol)。将反应混合物在65℃下加热4小时,冷却至室温后,用乙酸乙酯(EtOAc,5 × 30 mL)萃取。合并有机相,依次用1N盐酸(2 × 30 mL)和水(40 mL)洗涤,随后用无水硫酸钠干燥并浓缩,得到固体产物。通过过滤收集固体,依次用1N盐酸(4 × 10 mL)和水(20 mL)洗涤,最后在50℃的真空烘箱中干燥,得到N-Boc-4-氨基-2,6-二氯吡啶(7.44 g,收率93%)为白色固体。
参考文献:
[1] Patent: WO2015/76800, 2015, A1. Location in patent: Page/Page column 292
[2] Tetrahedron Letters, 2006, vol. 47, # 50, p. 8917 - 8920
[3] Journal of Organic Chemistry, 2008, vol. 73, # 15, p. 6025 - 6028
[4] Patent: WO2016/6974, 2016, A2. Location in patent: Paragraph 516; 517; 518; 519; 520; 521; 522
