502496-33-5
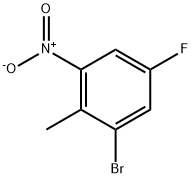 502496-33-5 结构式
502496-33-5 结构式
基本信息
5-溴-4-氟-6-硝基甲苯
2-硝基-4-氟-6-溴甲苯
2-溴-4-氟-6-硝基甲苯
15、2-溴-4-氟-6-硝基甲苯
1-溴-5-氟-2-甲基-3-硝基苯
2-溴-4-氟-6-硝基甲苯 100G
苯, 1-溴-5-氟-2-甲基-3-硝基-
Bromo-4-fluoro-6-nit
Bromo-4-fluoro-6-nitrotoluene
2-BROMO-4-FLUORO-6-NITROTOLUENE
3-BroMo-5-fluoro-2-Methylnitrobenzene
1-bromo-5-fluoro-2-methyl-3-nitrobenzene
Benzene, 1-broMo-5-fluoro-2-Methyl-3-nitro-
1-bromo-5-fluoro-2-methyl-3-nitrobenzene,2-Bromo-4-fluoro-6-nitrotoluene
3-Bromo-5-fluoro-2-methylnitrobenzene, 1-Bromo-5-fluoro-2-methyl-3-nitrobenzene
物理化学性质
制备方法
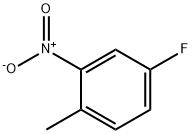
446-10-6

502496-33-5
步骤23a:将3-溴-5-氟-2-甲基苯胺(化合物0104-69)加入至含有4-氟-2-硝基甲苯(10.0g,64.4mmol)的三氟乙酸(40mL)溶液中。随后,缓慢加入硫酸(12.5mL),并分批加入N-溴代琥珀酰亚胺(NBS,17.2g,96.6mmol)。将反应混合物于室温下搅拌反应16小时。反应完成后,将混合物缓慢倒入冰水中,继续搅拌15分钟。用乙酸乙酯进行萃取,合并有机层,用饱和食盐水洗涤,无水硫酸钠干燥,减压浓缩,得到目标产物1-溴-5-氟-2-甲基-3-硝基苯(15.0g,100%收率),为黄色油状物。产物经1H NMR(400MHz,DMSO-d6)表征:δ2.41(s,3H),7.96(dd,J = 8.0,2.4Hz,1H),8.02(dd,J = 8.0,2.4Hz,1H)。
参考文献:
[1] Patent: WO2011/130628, 2011, A1. Location in patent: Page/Page column 155
[2] Patent: US2013/102595, 2013, A1. Location in patent: Paragraph 0279; 0280
[3] Patent: JP2015/187145, 2015, A. Location in patent: Paragraph 0252
[4] Patent: WO2007/122410, 2007, A1. Location in patent: Page/Page column 66
[5] Patent: WO2008/152387, 2008, A1. Location in patent: Page/Page column 33
