50765-11-2
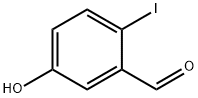 50765-11-2 结构式
50765-11-2 结构式
基本信息
5-羟基-2-碘苯甲醛
5-HYDROXY-2-IODOBENZALDEHYDE
Benzaldehyde, 5-hydroxy-2-iodo-
物理化学性质
制备方法

100-83-4

50765-11-2
在室温下,将1.0 g (8.18 mmol) 3-羟基苯甲醛溶解于100 mL二氯甲烷中,缓慢加入1.078 g (12.28 mmol) HBF4·OEt2,随后加入3.045 g (8.18 mmol) IPy2BF4(双(吡啶基)碘鎓(I)四氟硼酸盐)。反应混合物在室温下搅拌5分钟后,加入15 mL 1M HCl水溶液。水相用二氯甲烷萃取(3×10 mL),合并有机相,依次用10 mL水和25 mL 5%硫代硫酸钠水溶液洗涤,无水硫酸钠干燥。减压浓缩除去溶剂,得到褐色固体。该固体通过氯仿重结晶纯化,得到443 mg (22% yield) 5-羟基-2-碘苯甲醛,为褐色固体,熔点130-132°C(点燃熔点125-126°C)。 1H NMR (300 MHz, CD3COCD3): δ 9.96 (s, 1H), 9.15 (s, 1H), 7.85 (d, J = 8.6 Hz, 1H), 7.34 (d, J = 3.1 Hz, 1H), 6.98 (dd, J1 = 8.5 Hz, J2 = 3.1 Hz, 1H); 1H NMR (300 MHz, CDCl3): δ 9.99 (s, 1H), 7.79 (d, J = 8.4 Hz, 1H), 7.45 (d, J = 3.0 Hz, 1H), 6.91 (dd, J1 = 8.4 Hz, J2 = 3.0 Hz, 1H), 6.18 (br s, 1H); 13C NMR (75 MHz, CD3COCD3): δ 196.6 (CHO), 160.1 (C-5), 143.3 (C-3), 137.9 (C-1), 125.4 (C-4), 118.1 (C-6), 88.5 (C-2); 13C NMR (75 MHz, CDCl3): δ 196.3 (CHO), 156.7 (C-5), 141.4 (C-3), 135.7 (C-1), 123.9 (C-4), 116.5 (C-6), 89.4 (C-2); HRMS (EI) m/z: [M]+ calcd for C7H5IO2: 247.9334; found: 247.9292.
参考文献:
[1] Organic Preparations and Procedures International, 2017, vol. 49, # 3, p. 265 - 272
[2] Journal of the Chemical Society, 1937, p. 76
[3] Journal of the Indian Chemical Society, 1952, vol. 29, p. 363,364
[4] Journal of Medicinal Chemistry, 1973, vol. 16, p. 684 - 687
[5] Patent: WO2016/55947, 2016, A1. Location in patent: Page/Page column 70
