518044-32-1
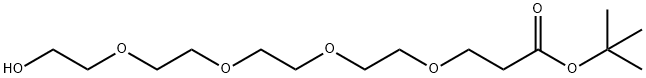 518044-32-1 结构式
518044-32-1 结构式
基本信息
丙酸叔丁酯-四聚乙二醇
四聚乙二醇-丙酸叔丁酯
羟基-PEG4-叔丁酯
羟基-四乙二醇-丙酸叔丁酯
羟基四聚乙二醇缬氨酸叔丁酯
羟基-四聚乙二醇-丙酸叔丁酯
丙酸叔丁酯-四聚乙二醇 1G
1-羟基-3,6,9,12-四氧杂十五烷酸叔丁酯
1-羟基-3,6,9,12-四氧杂十五烷-15-酸叔丁酯
518044-32-1
HO-PEG4-COOtBu
PEG5-t-butly ester
HO-PEG4-CH2CH2COOTBU
OH-PEG4-CH2CH2COOtBu
Hydroxy-PEG4-(CH2)2-Boc
HO-PEG4 tert-Butyl Ester
Hydroxy-PEG4-t-butly ester
tert-Butyl PEG4 propanoate
物理化学性质
制备方法
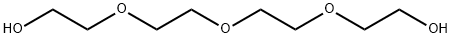
112-60-7
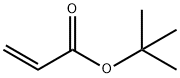
1663-39-4

518044-32-1
一般步骤:向三缩四乙二醇(40.61 mL; 45.64 g; 235 mmol)的无水四氢呋喃(THF,125 mL)溶液中加入一片钠(约1/4 cm)。待钠完全反应后,在20分钟内逐滴加入丙烯酸叔丁酯(11.98 mL; 10.57 g; 82.5 mmol),并将反应混合物在室温下搅拌过夜。反应完成后,用1N NaOH溶液调节pH至7-8,随后在减压下除去溶剂。将残余物溶解于饱和NaHCO3和NaCl混合溶液(75 mL)中,并用乙酸乙酯(EtOAc,3×100 mL)萃取。合并有机层,用无水硫酸镁(MgSO4)干燥,过滤后减压蒸发溶剂,得到叔丁基-15-羟基-4,7,10,13-四氧杂十五烷酸酯(21.87 g; 67.8 mmol; 产率82%,基于丙烯酸叔丁酯计算),为无色油状物。产物经1H-NMR(500 MHz, CDCl3, TMS)和13C{1H}-NMR(126 MHz, CDCl3, TMS)表征,数据如下:1H-NMR δ [ppm] = 3.77-3.57 (m, 18H, OCH2); 3.01 (bs, 1H, OH); 2.51 (t, 2H, J = 6.6 Hz, CH2COO'Bu); 1.45 (s, 9H, C(CH3)3); 13C{1H}-NMR δ [ppm] = 171.1 (COO); 80.7 (C(CH3)3); 72.6 / 70.8 / 70.7 / 70.6 / 70.5 / 67.0 (OCH2); 61.9 (HOCH2); 36.4 (CH2CO); 28.2 (C(CH3)3)。
参考文献:
[1] Synthetic Communications, 2004, vol. 34, # 13, p. 2425 - 2432
[2] Patent: WO2016/146638, 2016, A1. Location in patent: Page/Page column 20
[3] Patent: WO2017/147542, 2017, A2. Location in patent: Paragraph 00162
[4] Patent: WO2018/89373, 2018, A2. Location in patent: Paragraph 0638-0639
[5] Tetrahedron, 2011, vol. 67, # 12, p. 2251 - 2259
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-W039178 | 丙酸叔丁酯-四聚乙二醇 Hydroxy-PEG4-(CH2)2-Boc | 518044-32-1 | 1 g | 185元 |
| 2025/12/22 | HY-W039178 | 丙酸叔丁酯-四聚乙二醇 Hydroxy-PEG4-(CH2)2-Boc | 518044-32-1 | 5 g | 740元 |
| 2025/12/22 | HY-W039178 | 丙酸叔丁酯-四聚乙二醇 Hydroxy-PEG4-(CH2)2-Boc | 518044-32-1 | 10 g | 1335元 |
常见问题列表
|
Non-cleavable
|
Alkyl/ether
|
PEGs
|
ADCs are comprised of an antibody to which is attached an ADC cytotoxin through an ADC linker.
