52804-26-9
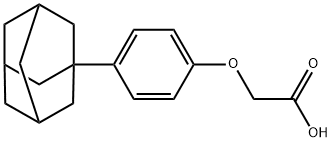 52804-26-9 结构式
52804-26-9 结构式
基本信息
2-[4-(1-金刚烷基)苯氧乙酸
[4-(金刚烷基-1)苯氧基]乙酸
[4-(1-金刚烷基)苯氧基]乙酸
2-(4-(1-金刚烷)苯氧基)乙酸
2-[4-(1-金刚烷基)苯氧基]乙酸
2-(4-(金刚烷-1-基)苯氧基)乙酸
BAS 00285042
Oprea1_723072
Oprea1_157400
CBDivE_002637
[4-(Adamantyl-1)phenoxy]acetic acid
(4-AdaMantan-1-yl-phenoxy)-aceticacid
2-[4-(1-adamantyl)phenoxy]acetic acid
2-[4-(1-adamantyl)phenoxy]ethanoic acid
2-(4-(adaMantan-1-yl)phenoxy)acetic acid
物理化学性质
制备方法
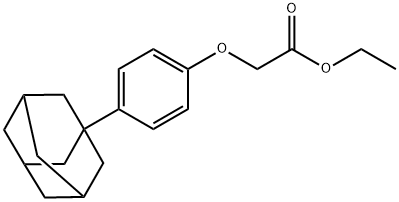
52804-25-8

52804-26-9
向2-(4-(1-金刚烷)苯氧基)乙酸乙酯(1.10 g,3.50 mmol)的THF/H2O(1:1,20.0 mL)溶液中加入LiOH·H2O(0.29 g,7.00 mmol)。将反应混合物在室温下搅拌过夜。反应完成后,用10% HCl溶液酸化至pH 2。加入EtOAc萃取,分离有机层。浓缩有机相后,通过硅胶柱色谱法(洗脱剂:CH2Cl2/MeOH = 30:1)纯化残余物,得到2-(4-(1-金刚烷)苯氧基)乙酸,为白色固体(0.61 g,收率61.3%)。1H NMR (CDCl3, 500 MHz) δ 7.29 (d, 2H, J = 6.75 Hz, Ar-H), 6.87 (d, 2H, J = 6.74 Hz, Ar-H), 4.61 (s, 2H, -OCH2COO-), 2.04 (s, 4H, 金刚烷基), 1.86 (m, 6H, 金刚烷基), 1.76 (m, 6H, 金刚烷基); 13C NMR (CDCl3, 125 MHz) δ 156.16, 142.71, 127.35, 114.31, 64.21, 42.51, 36.84, 36.17, 28.16; LCMS m/z 计算值 (MH+) 287.16,实测值 287.26。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 22, p. 6305 - 6310
[2] Journal of Medicinal Chemistry, 2007, vol. 50, # 7, p. 1675 - 1684
[3] Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 24, p. 7456 - 7460
[4] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 4, p. 1453 - 1456
[5] Bulletin of the Korean Chemical Society, 2010, vol. 31, # 12, p. 3826 - 3829