5308-63-4
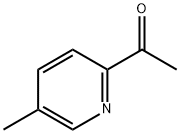 5308-63-4 结构式
5308-63-4 结构式
基本信息
1-(5-甲基吡啶-2-基)乙酮
2-乙酰基-5-甲基吡啶1-(5-METHYLPYRIDIN-2-YL)ETHANONE
1-(5-Methyl-2-pyridinyl)-Ethanone
1-(5-METHYL-PYRIDIN-2-YL)-ETHANONE
Ethanone, 1-(5-methyl-2-pyridinyl)-
1-(5-methylpyridin-2-yl)ethan-1-one
1-(5-methyl-2-pyridinyl)ethanone(SALTDATA: HCl)
1-(5-METHYL-PYRIDIN-2-YL)-ETHANONE ISO 9001:2015 REACH
物理化学性质
制备方法
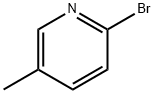
3510-66-5
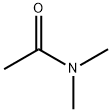
127-19-5

5308-63-4
以2-溴-5-甲基吡啶和N,N-二甲基乙酰胺为原料合成2-乙酰基-5-甲基吡啶的一般步骤:[参考例35] 5-(5-甲基-2-吡啶基)-1-(2-吡啶基)-1H-吡唑-3-羧酸; [显示图像] 1) 1-(5-甲基-2-吡啶基)乙酮; 在-78℃下,于5分钟内将正丁基锂(1.58M己烷溶液,24ml)滴加至2-溴-5-甲基吡啶(5.0g)的二乙醚(100ml)溶液中,搅拌混合物5分钟。保持相同温度,将N,N-二甲基乙酰胺(3.5ml)加入反应液中,继续搅拌2小时。反应完成后,向反应液中加入水和乙酸乙酯,分离有机相和水相。有机层用无水硫酸镁干燥,过滤后,减压浓缩除去溶剂。残余物通过硅胶柱色谱法(洗脱剂:己烷-乙酸乙酯)纯化,得到1-(5-甲基-2-吡啶基)乙酮(3.43g,收率87%),为油状产物。1H-NMR(400MHz, CDCl3) δ: 2.42(3H, s), 2.71(3H, s), 7.62(1H, dd, J = 1.59, 7.93Hz), 7.94(1H, d, J = 7.93Hz), 8.50(1H, s)。
参考文献:
[1] Inorganic Chemistry, 2018,
[2] Patent: EP1698626, 2006, A1. Location in patent: Page/Page column 48
[3] Patent: EP1762568, 2007, A1. Location in patent: Page/Page column 30
[4] Patent: EP1785418, 2007, A1. Location in patent: Page/Page column 38
[5] Chemistry - A European Journal, 2008, vol. 14, # 2, p. 570 - 581
