53277-47-7
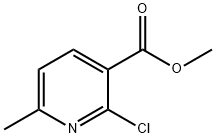 53277-47-7 结构式
53277-47-7 结构式
基本信息
2-氯-6-甲基烟酸甲酯
2-氯-6-甲基吡啶-3-羧酸甲酯
Methyl 2-chloro-6-methylnicotinate
METHYL6-METHYL-2-CHLOROPYRIDINE-3-CARBOXYLATE
2-Chloro-6-methyl-3-pyridinecarboxylic acid methyl ester
3-Pyridinecarboxylic acid, 2-chloro-6-Methyl-, Methyl ester
Methyl 2-chloro-6-methylpyridine-3-carboxylate, 2-Chloro-3-(methoxycarbonyl)-6-methylpyridine
物理化学性质
制备方法

67-56-1
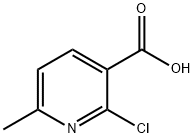
30529-70-5

53277-47-7
以甲醇和2-氯-6-甲基烟酸为原料合成2-氯-6-甲基吡啶-3-羧酸甲酯的一般步骤如下:a. 将草酰氯(9.3 mL)缓慢加入含有2-氯-6-甲基烟酸(9 g)的二氯甲烷(500 mL)溶液中。反应混合物在室温下搅拌30分钟后,通过减压浓缩去除溶剂,得到粗产物。随后,将粗产物溶解于甲醇(500 mL)中,并在0℃下搅拌处理。反应混合物在室温下继续搅拌过夜后,再次通过减压浓缩去除溶剂,得到残余物。将残余物用水和乙酸乙酯(EA)稀释,用饱和碳酸氢钠水溶液中和,随后用乙酸乙酯萃取三次。合并有机层,用盐水洗涤,经无水硫酸钠干燥,过滤后减压浓缩,得到2-氯-6-甲基吡啶-3-羧酸甲酯(9.7 g,收率99%)。产物经1H NMR(400 MHz,氯仿-d)表征:δ 2.60(s,3H),3.95(s,3H),7.17(d,J = 7.8 Hz,1H),8.09(d,J = 7.8 Hz,1H)。
参考文献:
[1] Patent: US2011/152312, 2011, A1. Location in patent: Page/Page column 35
[2] Patent: WO2009/100171, 2009, A1. Location in patent: Page/Page column 82
[3] Patent: US2010/144760, 2010, A1. Location in patent: Page/Page column 30; 31
[4] Patent: WO2010/72722, 2010, A1. Location in patent: Page/Page column 50
[5] Patent: WO2006/25783, 2006, A1. Location in patent: Page/Page column 65-66
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW0288637205004 | 4-氯-6-甲基烟酸甲酯 Methyl 4-chloro-6-methylnicotinate | 53277-47-7 | 5g | 505元 |
| 2025/12/22 | XW0288637205003 | 4-氯-6-甲基烟酸甲酯 Methyl 4-chloro-6-methylnicotinate | 53277-47-7 | 1g | 127元 |
| 2025/12/22 | XW0288637205002 | 4-氯-6-甲基烟酸甲酯 Methyl 4-chloro-6-methylnicotinate | 53277-47-7 | 250mg | 59元 |
