54123-21-6
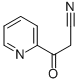 54123-21-6 结构式
54123-21-6 结构式
基本信息
3-氧代-3-(2-吡啶基)丙腈
b-oxo-2-Pyridinepropanenitrile
2-Pyridinepropanenitrile, β-oxo-
3-(2-PYRIDYL)-3-OXOPROPANENITRILE
3-OXO-3-(2-PYRIDYL)PROPANENITRILE
Beta-oxo-2-pyridinepropanenitrile
3-OXO-3-PYRIDIN-2-YL-PROPIONITRILE
3-oxo-3-pyridin-2-yl-propionatrile
3-OXO-2-PYRIDIN-3-YL-PROPIONITRILE
3-Oxo-3-pyridin-2-ylpropanenitrile
物理化学性质
制备方法
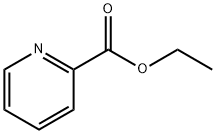
2524-52-9

75-05-8

54123-21-6
中间体35的制备[通用程序14]: 在干燥条件下,将中间体34(3g,19.6mmol)和乙腈(0.8mL,19.6mmol,1当量)溶于甲苯(10mL)中。将此溶液缓慢加入至预先在甲苯(50mL)中悬浮的氢化钠(784mg,19.6mmol,1当量,60%矿物油分散体)混合物中,反应温度维持在65℃。反应混合物在65℃下持续搅拌16小时。反应完成后,将混合物冷却至室温,并用冰冷的水(20mL)淬灭反应。通过过滤收集生成的固体,得到中间体35(1.5g,产率53%),为棕色固体。产物经1H NMR(CDCl3)鉴定: δ 8.70(d, J = 4.8Hz, 1H), 8.12(d, J = 7.5Hz, 1H), 7.90-7.94(m, 1H), 7.56-7.60(m, 1H), 4.41(s, 2H)。质谱分析显示分子离子峰[M + H]+为147。薄层色谱(TLC)分析,展开剂为乙酸乙酯,Rf值为0.40。
参考文献:
[1] Tetrahedron Letters, 2006, vol. 47, # 15, p. 2531 - 2534
[2] Patent: WO2011/126903, 2011, A2. Location in patent: Page/Page column 100
[3] Patent: WO2014/145986, 2014, A1. Location in patent: Paragraph 0267; 0268
[4] Patent: US2017/326125, 2017, A1. Location in patent: Paragraph 0406-0408
[5] Patent: US2014/179676, 2014, A1. Location in patent: Paragraph 0898; 0908
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW025412321604 | 3-氧代-3-(2-吡啶基)丙腈 3-Oxo-3-(2-pyridinyl)propanenitrile | 54123-21-6 | 10g | 1472元 |
| 2025/12/22 | XW025412321602 | 3-氧代-3-(2-吡啶基)丙腈 3-Oxo-3-(2-pyridinyl)propanenitrile | 54123-21-6 | 1g | 300元 |
| 2025/12/22 | XW025412321601 | 3-氧代-3-(2-吡啶基)丙腈 3-Oxo-3-(2-pyridinyl)propanenitrile | 54123-21-6 | 250mg | 145元 |
