56187-04-3
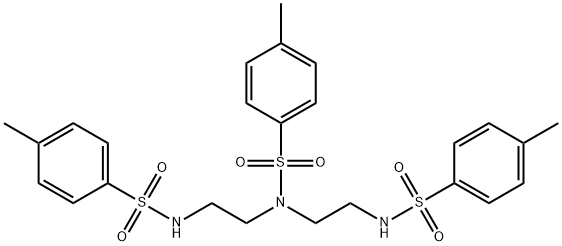 56187-04-3 结构式
56187-04-3 结构式
基本信息
N,N',N''-三磺酰二乙烯三胺
N,N',N''-三甲苯磺酰二乙烯三胺
N,N',N''-三(对甲苯磺酰)二乙撑三胺
N,N',N''-三(对甲苯磺酰)二乙烯三胺
N,N',N''-三(对甲苯磺酰基)二乙烯三胺
N,N',N''-三(对甲苯磺酰)二乙撑三胺
Gadobutrol Impurity 42
N,N',N''-TRITOSYLDIETHYLENETRIAMINE
N,N',N''-TRI-P-TOSYLDIETHYLENETRIAMINE, 98%
N,N',N''-TRIS(P-TOLUENESULFONYL)DIETHYLENETRIAMINE
N,N',N''-TRIS(P-TOLUENESULFIONYL)DIETHYLENETRIAMINE
N N N'-TRIS(P-TOLUENESULFONYL)DIETHYLENETRIAMINE 98+%
N-ethyl-2-methyl-N-(2-methylphenyl)sulfonylbenzenesulfonamide
4-Methyl-N,N-bis(2-(4-MethylphenylsulfonaMido)ethyl)benzenesulfonaMide
4-methyl-N,N-bis(2-(((4-methylphenyl)sulfonyl)amino)ethyl)benzenesulfonamide
物理化学性质
制备方法
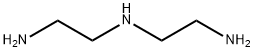
111-40-0

98-59-9

56187-04-3
在室温下,将115g(0.6mol)对甲苯磺酰氯溶解于250ml吡啶中。缓慢滴加含有二乙烯三胺(21.5ml,0.2mol)的吡啶溶液(30ml)。将反应混合物在50℃下持续搅拌90分钟,随后在温热状态下转移至锥形瓶内。待混合物冷却至室温后,加入200ml水。将混合物在室温下搅拌过夜,随后置于冰浴中冷却2小时。过滤收集形成的橙色固体,并用预冷至0℃的95%乙醇洗涤。最后,将产物在40℃下通过旋转蒸发器进行真空干燥,得到浅黄色固体状的目标化合物N,N,N-三(对甲苯磺酰基)二乙烯三胺(92g,收率81%)。
参考文献:
[1] Organic and Biomolecular Chemistry, 2007, vol. 5, # 22, p. 3651 - 3656
[2] Zeitschrift fur Naturforschung - Section B Journal of Chemical Sciences, 2007, vol. 62, # 3, p. 397 - 406
[3] Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2002, vol. 41, # 2, p. 372 - 375
[4] Dalton Transactions, 2016, vol. 45, # 23, p. 9412 - 9418
[5] Journal of the American Chemical Society, 2010, vol. 132, # 49, p. 17366 - 17369
