62458-96-2
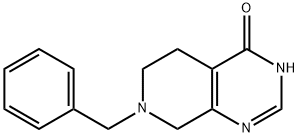 62458-96-2 结构式
62458-96-2 结构式
基本信息
7-苄基-5,6,7,8-四氢吡啶并[3,4-D]嘧啶-4(3H)-酮
7-苄基-5,6,7,8-T四氢吡啶并[3,4-D]嘧啶-4(3H)-酮
7-苄基-5,6,7,8-四氢吡啶并[3,4-D]嘧啶-4(4AH)-酮
7-苄基-5,6,7,8-T四氢吡啶并[3,4-D]嘧啶-4(3H)-酮 1G
7-benzyl-5
4-d]pyriMidin-4(4aH)-one
7-Benzyl-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4(3H)
7-benzyl-3H,4H,5H,6H,7H,8H-pyrido[3,4-d]pyriMidin-4-one
7-benzyl-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4(3H)-one
5,6,7,8-Tetrahydro-7-(phenylmethyl)pyrido[3,4-d]pyrimidin-4(
7-benzyl-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4(4aH)-one
7-Benzyl-5,6,7,8-tetrahydro-4aH-pyrido[3,4-d]pyrimidin-4-one
7-BENZYL-5,6,7,8-TETRAHYDROPYRIDO[3,4-D]PYRIMIDIN-4(3H)-ONE HCL
物理化学性质
制备方法
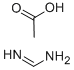
3473-63-0
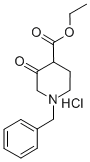
52763-21-0
![7-苄基-5,6,7,8-T四氢吡啶并[3,4-D]嘧啶-4(3H)-酮](/CAS/GIF/62458-96-2.gif)
62458-96-2
在25℃条件下,向甲醇钠(25wt%甲醇溶液,67.6mL,296mmol)和甲醇(70mL)的混合溶液中依次加入醋酸甲脒(11.00g,106mmol)及1-苄基-3-氧代哌啶-4-甲酸乙酯盐酸盐(25.16g,84mmol)。将反应混合物于25℃持续搅拌20小时。反应完成后,将混合物冷却至0℃,加入水(90mL),随后缓慢滴加乙酸(6.05mL,106mmol),并于25℃继续搅拌3小时。通过减压蒸馏去除大部分甲醇,浓缩反应混合物。过滤所得悬浮液,收集固体产物,用水洗涤后,于真空下干燥,得到7-苄基-5,6,7,8-四氢吡啶并[3,4-d]嘧啶-4(3H)-酮(16.10g,收率79%)为白色固体。LC/MS分析显示:m/z 242.06([M+H]+),保留时间0.598分钟(方法12)。1H-NMR(500MHz,CDCl3)δ12.61(宽单峰,1H),7.99(单峰,1H),7.38-7.26(多重峰,5H),3.73(多重峰,2H),3.50(多重峰,2H),2.74(多重峰,2H),2.66(多重峰,2H)。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2016, vol. 26, # 1, p. 160 - 167
[2] Patent: WO2009/158396, 2009, A1. Location in patent: Page/Page column 67
[3] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 18, p. 5019 - 5024
[4] Patent: US2008/275052, 2008, A1. Location in patent: Page/Page column 45
