642494-37-9
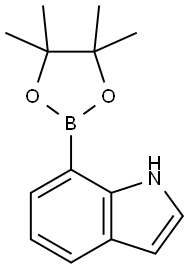 642494-37-9 结构式
642494-37-9 结构式
基本信息
吲哚-7-硼酸频哪醇酯
1H-Indole-7-boronic acid, pinacol ester
1H-INDOL-7-YLBORONIC ACID PINACOL ESTER
REF DUPL: Indole-7-boronic acid pinacol ester
7-(tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-indole
4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-3H-indole
7-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-1H-INDOLE
1H-Indole, 7-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-
3-hydroxy-2,3-dimethylbutan-2-yl hydrogen 1H-indol-7-ylboronate
7-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indole, 2-(1H-Indol-7-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
物理化学性质
制备方法
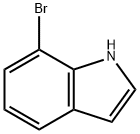
51417-51-7

73183-34-3

642494-37-9
以7-溴吲哚和联硼酸频那醇酯为原料合成7-吲哚硼酸频那醇酯的一般步骤如下:将7-溴吲哚(20g,0.102mol)溶解于装有DMSO(100mL)的1L单颈烧瓶中,依次加入双(频哪醇合)二硼(39g,0.153mol)、[1,1'-双(二苯基膦基)二茂铁]二氯钯(Pd(dppf)Cl2,4g,0.005mol)和乙酸钾(15g,0.153mol)。将反应体系加热至85℃并搅拌反应2小时。反应完成后,加入水淬灭反应。用乙酸乙酯对混合物进行三次萃取,合并有机相并用无水硫酸钠干燥。减压浓缩后,通过柱色谱法纯化,得到目标产物7-吲哚硼酸频那醇酯(25g,0.103mol)。产物的结构通过1H NMR(400MHz,CDCl3)和质谱(ESI)确认:1H NMR (400 MHz, CDCl3) δ 9.29 (s, 1H), 7.82 (d, J = 7.9 Hz, 1H), 7.70 (d, J = 7.0 Hz, 1H), 7.32-7.29 (m, 1H), 7.21-7.14 (m, 1H), 6.62-6.57 (m, 1H), 1.44 (s, 12H); MS (ESI) m/z: [M + H]+ 244.1。
参考文献:
[1] Synlett, 2003, # 8, p. 1204 - 1206
[2] Advanced Synthesis and Catalysis, 2017, vol. 359, # 19, p. 3421 - 3427
[3] Journal of the American Chemical Society, 2017, vol. 139, # 24, p. 8267 - 8276
[4] European Journal of Organic Chemistry, 2016, vol. 2016, # 27, p. 4621 - 4628
[5] Patent: JP2015/528445, 2015, A. Location in patent: Paragraph 0047; 0065
