66491-03-0
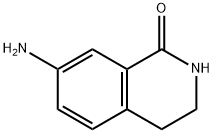 66491-03-0 结构式
66491-03-0 结构式
基本信息
7-氨基-3,4-二氢异喹啉-1(2H)-酮
7-氨基-3,4-二氢-2H-异喹啉-1-酮
66491-03-0 7-氨基-3,4-二氢异喹啉-1(2H)-酮
7-AMINO-3,4-DIHYDRO-2H-ISOQUINOLIN-1-ONE
7-Amino-3,4-dihydro-1(2H)-isoquinolinone
1(2H)-Isoquinolinone, 7-aMino-3,4-dihydro-
2-Propenoicacid,2-methyl-,2-(4-hydroxyethoxy)ethylester
物理化学性质
制备方法
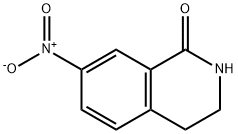
22245-96-1

66491-03-0
以7-硝基-3,4-二氢异喹啉-1(2H)-酮为原料合成7-氨基-3,4-二氢异喹啉-1(2H)-酮的一般步骤:将7-硝基-3,4-二氢异喹啉-1(2H)-酮(700mg,3.6mmol)溶于甲醇(25mL)中,加入10%钯/碳催化剂(催化量)。在氢气(60psi)氛围下搅拌反应1小时。反应完成后,将混合物通过硅藻土过滤以去除催化剂,滤液经真空浓缩得到7-氨基-3,4-二氢异喹啉-1(2H)-酮(500mg,收率86%)。产物经1H NMR(400MHz,CD3OD)表征:δ 2.81(t,J = 6.59Hz,2H),3.42(t,J = 6.55Hz,2H),6.84(dd,J = 8.13,2.42Hz,1H),7.02(d,J = 7.91Hz,1H),7.26(d,J = 2.64Hz,1H)。
参考文献:
[1] Journal of Organic Chemistry, 1983, vol. 48, # 19, p. 3220 - 3234
[2] Patent: WO2008/79759, 2008, A1. Location in patent: Page/Page column 98
[3] Patent: WO2008/79836, 2008, A2. Location in patent: Page/Page column 86
[4] Journal of Medicinal Chemistry, 2015, vol. 58, # 6, p. 2799 - 2808
[5] ACS Medicinal Chemistry Letters, 2016, vol. 7, # 12, p. 1077 - 1081
