6683-46-1
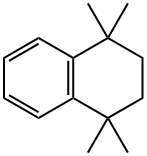 6683-46-1 结构式
6683-46-1 结构式
基本信息
1,1,4,4-四甲基-1,2,3,4-四氢萘
1,2,3,4-四氢-1,1,4,4-四甲基萘
1,1,4,4-四甲基-1,2,3,4-四氢化萘
1,1,4,4-Tetramethyltetralin
1,1,4,4-tetramethyl-2,3-dihydronaphthalene
1,1,4,4-TETRAMETHYL-1,2,3,4-TETRAHYDRONAPHTHALENE
1,2,3,4-TETRAHYDRO-1,1,4,4-TETRAMETHYLNAPHTHALENE
1,2,3,4-tetrahydro-1,1,4,4-tetramethyl-naphthalen
naphthalene,1,2,3,4-tetrahydro-1,1,4,4-tetramethyl-
1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthaline, tech
1,2,3,4-Tetrahydro-1,1,4,4-tetramethylnaphthalene, 97+%
1,1,4,4-Tetramethyl-1,2,3,4-tetrahydronaphthalene, Tech.
物理化学性质
安全数据
制备方法
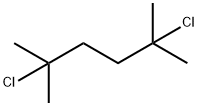
6223-78-5
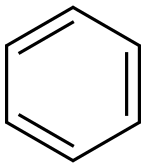
71-43-2

6683-46-1
以2,5-二氯-2,5-二甲基己烷和苯为原料合成1,1,4,4-四甲基-1,2,3,4-四氢萘的一般步骤:将2,5-二氯-2,5-二甲基己烷(300 mg,1.64 mmol,1当量)溶解于干燥的苯(25 mL)中。向该溶液中加入无水三氯化铝(AlCl3,22.0 mg,0.164 mmol,0.1当量),并在回流条件下搅拌反应混合物16小时。反应完成后,用3M盐酸(5 mL)淬灭反应,并用己烷(10 mL × 3)进行萃取。合并有机层,用饱和食盐水洗涤,无水硫酸钠(Na2SO4)干燥,过滤后真空浓缩。通过快速柱色谱法(100%己烷作为洗脱剂)纯化粗产物,得到1,1,4,4-四甲基-1,2,3,4-四氢萘(309 mg,收率91%),为无色油状物。产物表征数据如下:1H NMR (500 MHz, CDCl3) δ 1.33 (s, 12H), 1.74 (s, 4H), 7.16-7.19 (m, 2H), 7.34-7.36 (m, 2H, J = 2 Hz和8.4 Hz);13C NMR (500 MHz, CDCl3) δ 31.9, 34.2, 35.1, 125.5, 126.5, 144.8。
参考文献:
[1] Patent: WO2015/188015, 2015, A1. Location in patent: Page/Page column 31
[2] Patent: WO2018/107289, 2018, A1. Location in patent: Page/Page column 17
[3] Journal of the Brazilian Chemical Society, 2018, vol. 29, # 1, p. 109 - 124
[4] Journal of Medicinal Chemistry, 1988, vol. 31, # 11, p. 2182 - 2192
[5] Bioorganic and Medicinal Chemistry, 2014, vol. 22, # 6, p. 1948 - 1959
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2017/11/01 | TL00201EA | 1,1,4,4-四甲基-1,2,3,4-四氢萘 1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthalene | 6683-46-1 | 10GR | 2645元 |
| 2017/11/01 | TL00201EE | 1,1,4,4-四甲基-1,2,3,4-四氢萘 1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthalene | 6683-46-1 | 50GR | 7310元 |
| 2017/11/01 | TL00201FA | 1,1,4,4-四甲基-1,2,3,4-四氢萘 1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthalene | 6683-46-1 | 100GR | 7904元 |
