67706-68-7
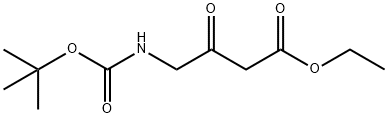 67706-68-7 结构式
67706-68-7 结构式
基本信息
4-(叔丁氧基羰基氨基)-3-氧代丁酸乙酯
4-(Boc-amino)-3-oxobutanoic acid ethyl ester
TERT-BUTYL 3-(ETHOXYCARBONYL)-2-OXOPROPYLCARBAMATE
Ethyl 4-((tert-butoxycarbonyl)aMino)-3-oxobutanoate
4-[(tert-Butoxycarbonyl)amino]-3-oxobutanoic acid ethyl ester
Butanoic acid, 4-[[(1,1-dimethylethoxy)carbonyl]amino]-3-oxo-, ethyl ester
物理化学性质
制备方法
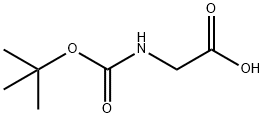
4530-20-5
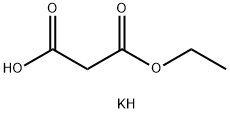
6148-64-7

67706-68-7
(1) 将羰基二咪唑(10.2 g,63.1 mmol)加入至2-(叔丁氧基羰基氨基)乙酸(10 g,57.1 mmol)的四氢呋喃(100 mL)悬浮液中,室温搅拌反应2小时。随后,向反应体系中依次加入氯化镁(5.4 g,57.1 mmol)和丙二酸单乙酯钾盐(9.7 g,57.1 mmol),并于60℃下继续搅拌1小时。反应完成后,过滤除去不溶物,滤液用乙酸乙酯(200 mL)稀释,依次以1N盐酸、饱和碳酸氢钠水溶液及饱和氯化钠水溶液洗涤,无水硫酸钠干燥,减压浓缩。所得残余物通过硅胶柱色谱纯化[洗脱剂:正己烷-乙酸乙酯(2:1)],得到4-(叔丁氧基羰基氨基)-3-氧代丁酸乙酯(11.9 g,48.5 mmol,收率85%)为无色油状物。1H-NMR (CDCl3) δ: 1.29 (3H, t, J = 7.4 Hz), 1.45 (2H, s), 3.87 (2H, s), 3.96 (3H, s), 4.21 (2H, q, J = 7.2 Hz), 7.08 (1H, d, J = 8.7 Hz), 7.39 (9H, s), 3.49 (2H, s), 4.14 (2H, d, J = 5.8 Hz), 4.21 (2H, q, J = 7.4 Hz), 5.15-5.24 (1H, br)。
参考文献:
[1] Organic Letters, 2005, vol. 7, # 2, p. 215 - 218
[2] Patent: EP1650201, 2006, A1. Location in patent: Page/Page column 23-24
[3] Patent: WO2016/118565, 2016, A1. Location in patent: Paragraph 00399
[4] Journal of Medicinal Chemistry, 2008, vol. 51, # 3, p. 487 - 501



