67938-76-5
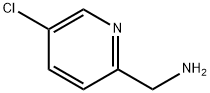 67938-76-5 结构式
67938-76-5 结构式
基本信息
2-氨甲基-5-氯吡啶
(5-氯吡啶-2-基)甲胺
2-氨甲基-5-氯吡啶盐酸盐
5-Chloro-2-pyridinemethanamine
2-Aminomethyl-5-chloropyridine
(5-chloro-2-pyridyl)methanamine
2-Pyridinemethanamine, 5-chloro-
1-(5-CHLOROPYRIDIN-2-YL)METHANAMINE
C-(5-Chloro-pyridin-2-yl)-MethylaMine
2-Aminomethyl-5-chloropyridine hydrochloride
1-(5-CHLOROPYRIDIN-2-YL)METHANAMINE ISO 9001:2015 REACH
物理化学性质
制备方法
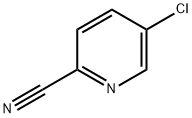
89809-64-3

67938-76-5
以5-氯-2-氰基吡啶为原料合成2-氨甲基-5-氯吡啶的一般步骤如下:将5-氯吡啶甲腈(3.8g,27.43mmol)溶于乙醇(100mL)中,加入浓HCl(3mL)和10%Pd-C催化剂(1.0g)。在氢气气氛(40psi)下振荡反应混合物2小时。反应完成后,过滤去除催化剂,浓缩滤液。将得到的残余物溶解于饱和NaHCO3溶液(50mL)中,用二氯甲烷(4×25mL)进行萃取。合并有机相,用无水Na2SO4干燥,过滤并浓缩,得到2-氨甲基-5-氯吡啶,为黄色油状物(2.0g,收率51%)。产物经LCMS分析显示(M + H)+峰值为143.07(计算值:143.04,C6H8ClN2)。1HNMR(500 MHz,CDCl3)δ ppm:8.56-8.51(1H,br d),7.66-7.60(1H,m),7.28-7.14(1H,m),3.97(2H,s),1.72(2H,s)。
参考文献:
[1] Patent: US2005/267105, 2005, A1. Location in patent: Page/Page column 63
[2] Patent: US2007/111984, 2007, A1. Location in patent: Page/Page column 29
[3] Patent: US2007/129379, 2007, A1. Location in patent: Page/Page column 21-22
[4] Patent: WO2007/64316, 2007, A1. Location in patent: Page/Page column 140 - 141
