683242-93-5
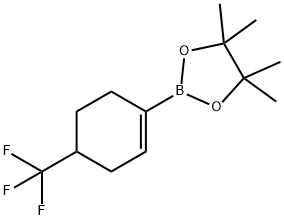 683242-93-5 结构式
683242-93-5 结构式
基本信息
4,4,5,5-四甲基-2-[4-(三氟甲基)-1-环己烯-1-基]-1,3,2-二氧杂环戊硼烷
4,4,5,5-Tetramethyl-2-[4-(trifluoromethyl)
4-(TrifluoroMethyl)cyclohex-1-enylboronic acid pinacol ester
4-(Trifluoromethyl)-1-cyclohexen-1-boronic acid pinacol ester
4-TRIFLUOROMETHYLCYCLOHEX-1-ENYL-1-BORONIC ACID PINACOL ESTER
4-Trifluoromethyl-1-cyclohexen-1-ylboronic acid pinacol ester
4-(Trifluoromethyl)-1-cyclohexene-1-boronic Acid Pinacol Ester
4,4,5,5-tetramethyl-2-[4-(trifluoromethyl)cyclohexen-1-yl]-1,3,2-dioxaborolane
4,4,5,5-Tetramethyl-2-[4-(trifluoromethyl)cyclohex-1-en-1-yl]-1,3,2-dioxaborolane
4,4,5,5-TetraMethyl-2-[4-(trifluoroMethyl)-1-cyclohexen-1-yl]-1,3,2-dioxaborolane
制备方法

865869-25-6

73183-34-3

683242-93-5
以4-(三氟甲基)环己-1-烯丙基三氟甲磺酸酯(2.24 g,7.50 mmol,1.00当量)和联硼酸频那醇酯(2.86 g,11.26 mmol,1.50当量)为原料,在氮气保护下,将Pd(dppf)Cl2(160 mg,0.22 mmol,0.03当量)、dppf(125 mg,0.23 mmol,0.03当量)和KOAc(2.2 g,22.42 mmol,2.99当量)溶于二恶烷(50 mL)中。将反应混合物在80℃下搅拌12小时。反应完成后,将混合物真空浓缩。通过硅胶柱色谱法纯化残余物,使用乙酸乙酯/石油醚(1/20)作为洗脱剂,得到4-(三氟甲基)-1-环己烯-1-硼酸频哪醇酯(1 g,产率48%)为白色固体。LCMS [M + H]+ 277。1H NMR (300 MHz, CDCl3) δ 6.53 (s, 1H), 2.37-1.98 (m, 7H), 1.26 (s, 12H)。
参考文献:
[1] Journal of Medicinal Chemistry, 2006, vol. 49, # 12, p. 3719 - 3742
[2] Journal of Medicinal Chemistry, 2007, vol. 50, # 15, p. 3528 - 3539
[3] Patent: WO2016/128529, 2016, A1. Location in patent: Paragraph 0828; 0831; 0832
[4] Patent: US2005/182067, 2005, A1. Location in patent: Page/Page column 28-29
[5] Patent: WO2014/49047, 2014, A1. Location in patent: Page/Page column 114; 115; 116
