6836-21-1
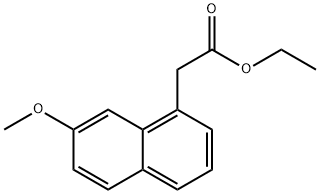 6836-21-1 结构式
6836-21-1 结构式
基本信息
7-甲氧基-1-萘乙酸乙酯
7-甲氧基-1-萘基乙酸乙酯
2-(7-甲氧基-1-萘基)乙酸乙酯
2-(7-甲氧基萘-1-基)乙酸乙酯
Ethyl 7-Methoxynaphthalene-1-acetate
Ethyl (7-Methoxynaphthalen-1-yl)acetate
Ethyl 2-(7-Methoxynaphthalen-1-yl)acetate
7-Methoxy-1-phthaleneacetic acid ethyl ester
7-Methoxy-1-naphthaleneacetic acid ethyl ester
1-Naphthaleneacetic acid, 7-methoxy-, ethyl ester
制备方法
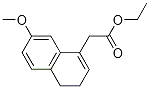
27533-70-6

6836-21-1
以阿戈美拉汀杂质21为原料合成7-甲氧基-1-萘基乙酸乙酯的一般步骤:向含有乙酸-2-(2-甲氧基萘-8-基)乙酸乙酯(3g,12.1mmol)的无水二氯甲烷(45mL,15体积)溶液中,在15℃下缓慢加入2,3-二氯-5,6-二氰基-1,4-苯醌(DDQ,4.15g,18.2mmol)。添加完毕后,将反应混合物在25-30℃下持续搅拌12小时。反应完成后,通过过滤分离混合物,沉淀的固体用二氯甲烷(30mL,10体积)洗涤。合并有机相,依次用饱和碳酸氢钠溶液(15mL,3×5体积)、水(15mL,3×5体积)和盐水(15mL,3×5体积)洗涤。有机层用无水硫酸钠干燥,随后在减压下浓缩除去溶剂,得到棕色粗产物。粗产物通过100-200目硅胶柱色谱纯化,以20%乙酸乙酯的正己烷溶液为洗脱剂,最终得到无色油状产物(2.59g,产率87.3%)。产物经IR(KBr,νmax,cm-1):2981,2937,2834,1733,1627,1601,1511,1469,1450,1260,1213,1159,133,832;1H NMR(400MHz,CDCl3):δ7.75(d,J=8.4Hz,1H),7.71(d,J=8.0Hz,1H),7.38(d,J=7.2Hz,1H),7.30(t,J=8.0Hz,2H),7.16(dd,J1=2.4Hz,J2=8.8Hz,1H),4.15(q,J=7.2Hz,2H),4.0(s,2H),3.93(s,3H),1.2(t,J=7.2Hz,3H);13C NMR(100Hz,DMSO-d6):δ171.1,157.46,132.99,130.01,129.79,128.74,128.37,127.25,123.02,117.88,117.88,102.76,60.23,54.94,14.0;EI-MS,m/z(百分比):245(M+1,100),171(17)进行表征确认。
参考文献:
[1] Organic Preparations and Procedures International, 2009, vol. 41, # 2, p. 164 - 168
[2] Asian Journal of Chemistry, 2016, vol. 28, # 6, p. 1367 - 1370
[3] Patent: CN104276979, 2018, B. Location in patent: Paragraph 0028; 0030; 0031

