686279-09-4
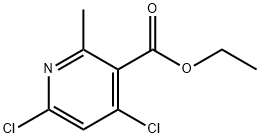 686279-09-4 结构式
686279-09-4 结构式
基本信息
4,6-二氯-2-甲基烟酸乙酯
4,6-二氯-2-甲基-3-吡啶羧酸乙酯
4,6-dichloro-2-Methyl-3-carbethoxypyridine
4,6-DICHLORO-2-METHYL-NICOTINIC ACID ETHYL ESTER
Ethyl 4,6-dichloro-2-methyl-pyridine-3-carboxylate
4,6-Dichloro-2-methylpyridin-3-carboxylic acid ethyl ester
4,6-Dichloro-2-methyl-3-pyridinecarboxylic acid ethyl ester
3-Pyridinecarboxylic acid, 4,6-dichloro-2-Methyl-, ethyl ester
物理化学性质
制备方法
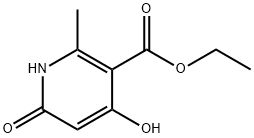
3950-10-5

686279-09-4
如方案9的步骤9-i所示,将4-羟基-2-甲基-6-氧代-1,6-二氢吡啶-3-羧酸乙酯(Accla Biochem Inc.)悬浮于50 mL的三氯氧磷(POCl3)中,在氮气保护下加热至90℃,反应5小时。反应完成后,冷却反应混合物,并在减压条件下浓缩。随后,在搅拌下向深色油状物中加入冰,接着加入乙酸乙酯和水进行萃取。有机相依次用水和饱和食盐水洗涤,经无水硫酸钠干燥。过滤后,减压除去溶剂,粗产物通过硅胶柱色谱法纯化,得到2-甲基-4,6-二氯烟酸乙酯(化合物2030),为浅黄色油状物,产率62%。质谱(ESMS)显示分子离子峰(M + H)为234/236/238;1H NMR(CDCl3)δ 7.27(s, 1H),4.40(q, 2H),2.55(s, 3H),1.41(t, 3H)。
参考文献:
[1] Patent: WO2011/87776, 2011, A1. Location in patent: Page/Page column 64; 66
[2] Patent: WO2007/16525, 2007, A2. Location in patent: Page/Page column 94-96; 101-103
[3] Patent: US2008/188467, 2008, A1. Location in patent: Page/Page column 26-27; 29-30; 40-41
[4] Patent: US2013/281397, 2013, A1. Location in patent: Paragraph 0451; 0465
[5] Patent: CN103319408, 2016, B. Location in patent: Paragraph 0402-0405
