71229-85-1
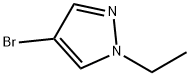 71229-85-1 结构式
71229-85-1 结构式
基本信息
1-乙基-4-溴吡唑
4-溴-1-乙基-1H-吡唑
-Bromo-1-ethylpyrazole
4-Bromo-1-ethylpyrazole
1H-Pyrazole,4-bromo-1-ethyl-
4-Bromo-1-ethyl-1H-pyrazole 95+%
4-bromo-1-ethyl-1H-pyrazole(SALTDATA: FREE)
物理化学性质
制备方法
2075-45-8

75-03-6

71229-85-1
a)4-溴-1-乙基-1H-吡唑的合成:在4-溴-1H-吡唑(5g,34mmol)的DMF溶液中加入碳酸钾(K2CO3,11.75g,85.03mmol,2.5当量)和碘乙烷(8g,51mmol,1.5当量)。将反应混合物在室温下搅拌12小时。反应完成后,按照中间体实施例5(c)的方法进行骤冷和萃取操作。通过蒸馏去除溶剂,得到的粗产物采用柱色谱法(60-120目硅胶,40%乙酸乙酯的己烷溶液作为洗脱剂)进行纯化,最终得到目标产物1-乙基-4-溴吡唑,收率为84%(5g)。产物结构通过1H NMR(300MHz,DMSO-d6)和LC-MS(ESI)进行确认:1H NMR (300MHz, DMSO-d6) δ 8.02 (s, 1H), 7.55 (s, 1H), 4.15 (q, 2H), 1.37 (t, 3H); LC-MS (ESI): 计算质量:175.03; 实测质量:177.0 [M + H]+(保留时间:0.56min)。
参考文献:
[1] Patent: WO2013/53983, 2013, A1. Location in patent: Page/Page column 36
[2] Patent: US2015/11548, 2015, A1. Location in patent: Paragraph 0150
[3] Patent: WO2015/92713, 2015, A1. Location in patent: Page/Page column 403
[4] Patent: WO2015/77503, 2015, A1. Location in patent: Paragraph 00341
[5] Patent: US9051320, 2015, B1. Location in patent: Paragraph 0258
