71999-74-1
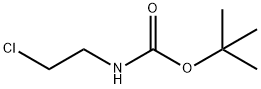 71999-74-1 结构式
71999-74-1 结构式
基本信息
N-BOC-2-氯乙胺
N-BOC-2-氯乙基胺
2-(BOC-氨基)乙基氯
N-BOC-2-氯乙胺 5G
(2-氯乙基)氨基甲酸 叔丁酯
N-(叔丁氧羰基)-2-氯乙基胺
N-Boc-2-Chloroethylamine
2-(Boc-amino)ethyl Chloride
tert-butyl 2-chloroethylcarbamate
2-Chloroethylamine, N-BOC protected
N-t-Butoxycarbonyl-2-chloroethylaMine
tert-Butyl N-(2-chloroethyl)carbamate
1-Amino-2-chloroethane, N-BOC protected
N-(tert-Butoxycarbonyl)-2-chloroethylaMine
(tert-butoxy)-N-(2-chloroethyl)-carboxaMide
物理化学性质
制备方法

24424-99-5
870-24-6

71999-74-1
在圆底烧瓶中,将2-氯乙胺盐酸盐(2 g,17.0 mmol)和三乙胺(Et3N,2.37 mL,11.0 mmol)溶解于无水二氯甲烷(CH2Cl2,24 mL)中。在氮气(N2)保护下,于0℃缓慢加入二碳酸二叔丁酯(Boc2O,3.6 mL,15.3 mmol)。反应混合物在室温下搅拌4小时。反应完成后,混合物依次用水(H2O)和盐水洗涤,无水硫酸钠(Na2SO4)干燥,过滤,减压浓缩,得到N-Boc-2-氯乙胺,为深色液体,产量2.91 g(16.21 mmol,收率99%),直接用于下一步反应。产物经红外光谱(IR,纯品)确认:3347, 2924, 2854, 2361, 2342, 1725, 1709, 1503, 1462 cm-1。核磁共振氢谱(1H NMR,400 MHz,CDCl3):δ=1.18 [s,9H,C(CH3)3],3.36-3.48(m,2H,CH2NH),3.49-3.63(m,2H,ClCH2),4.93(br s,1H,NHCO)。核磁共振碳谱(13C NMR,100 MHz,CDCl3):δ=28.2 [C(CH3)3],32.8(CH2NH),45.7(CH2Cl),79.8 [C(CH3)3],155.8(C=O)。元素分析(C7H14ClNO2)计算值:C,46.80;H,7.86;N,7.80。实测值:C,46.91;H,7.89;N,7.74。
参考文献:
[1] Journal of Medicinal Chemistry, 2014, vol. 57, # 18, p. 7731 - 7757
[2] Journal of Medicinal Chemistry, 2003, vol. 46, # 10, p. 1845 - 1857
[3] Synthesis (Germany), 2015, vol. 47, # 23, p. 3767 - 3775
[4] Journal of Polymer Science, Part A: Polymer Chemistry, 2018, vol. 56, # 19, p. 2154 - 2160
[5] Patent: WO2009/61131, 2009, A2. Location in patent: Page/Page column 81-82

