7251-53-8
中文名称
3-羟基异恶唑-4-甲酸乙酯
英文名称
ethyl 3-hydroxy-1H-pyrazole-4-carboxylate
CAS
7251-53-8
分子式
C6H8N2O3
分子量
156.14
MOL 文件
7251-53-8.mol
更新日期
2026/02/11 16:01:04
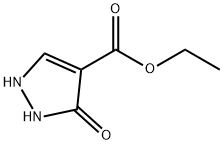 7251-53-8 结构式
7251-53-8 结构式
基本信息
中文别名
别嘌醇杂质 103-羟基吡唑-4-甲酸乙酯
3-羟基异噁唑-6-甲酸甲酯
3-羟基-吡唑-4-羧酸乙酯
3-羟基异恶唑-4-甲酸乙酯
3-羟基-1H-吡唑-4-羧酸乙酯
3-氧代-2,3-二氢-1H-吡唑-4-羧酸乙酯
3-氧代-2,3-二氢-1H-吡唑-4-甲酸乙酯
英文别名
Allopurinol Impurity 104-(Ethoxycarbonyl)-3-hydroxy-1H-pyrazole
ehyl 3-hydroxy-1H-pyrazole-4-carboxylate
ethyl 3-hydroxy-1H-pyrazole-4-carboxylate
Ethyl 5-hydroxy-1H-pyrazole-4-carboxylate
ethyl 3-oxo-1,2-dihydropyrazole-4-carboxylate
Ethyl 3-hydroxy-1H-pyrazole-4-carboxylate 95%
ethyl 3-oxo-2,3-dihydro-1H-pyrazole-4-carboxylate
2,3-dihydro-3-oxo-1H-Pyrazole-4-carboxylic acid ethyl ester
3-Oxo-2,3-dihydro-1H-pyrazole-4-carboxylic acid ethyl ester
所属类别
化学试剂:甲酯类化合物物理化学性质
熔点140-142 °C
密度1.272±0.06 g/cm3(Predicted)
储存条件Inert atmosphere,Room Temperature
酸度系数(pKa)7.78±0.70(Predicted)
外观White to yellow Solid
InChIInChI=1S/C6H8N2O3/c1-2-11-6(10)4-3-7-8-5(4)9/h3H,2H2,1H3,(H2,7,8,9)
InChIKeyPJFXDPZWCVXNQZ-UHFFFAOYSA-N
SMILESN1C=C(C(OCC)=O)C(=O)N1
制备方法
方法1
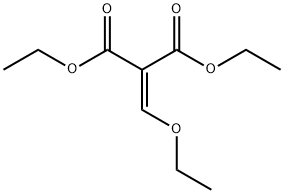
87-13-8

7251-53-8
3-氧代-2,3-二氢-1H-吡唑-4-羧酸乙酯的合成:在冰水浴冷却条件下,向乙醇钠(20.8 g,0.31 mol)和乙氧基亚甲基丙二酸二乙酯(20 mL,0.10 mol)的乙醇溶液(400 mL)中缓慢加入一水合肼(10.0 mL,0.20 mol)。随后,将反应混合物于80℃加热回流3小时。反应完成后,将混合物用水(200 mL)稀释,并用10 M HCl溶液调节pH至6。用氯仿进行多次萃取分离。水相进一步酸化至pH 2后,再次用氯仿萃取。合并所有有机相,用无水硫酸钠干燥,减压浓缩得到粗产物。粗产物经甲醇和乙醚洗涤后干燥,得到目标化合物3-氧代-2,3-二氢-1H-吡唑-4-羧酸乙酯(13.2 g,收率80%),为白色固体。产物经1H NMR(400 MHz,DMSO-d6)表征:δ 7.89(s,1H),4.15(q,J = 7.12 Hz,2H),1.23(t,J = 7.11 Hz,3H)。
参考文献:
[1] Patent: WO2009/89057, 2009, A1. Location in patent: Page/Page column 56
[2] Patent: WO2016/83816, 2016, A1. Location in patent: Page/Page column 48
[3] Patent: EP3272750, 2018, A1. Location in patent: Paragraph 0219; 0220
[4] Yakugaku Zasshi, 1957, vol. 77, p. 800
[5] Chem.Abstr., 1957, p. 17893
