73620-18-5
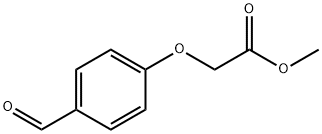 73620-18-5 结构式
73620-18-5 结构式
基本信息
(4-甲酰基苯氧基)乙酸甲酯
2-(4-甲酰苯氧基)乙酸甲酯
2-(4-甲酰基苯氧基)乙酸甲酯
2-(4-甲烷酰基苯氧基)乙酸甲酯
methyl (4-formylphenoxy)acetate
Methyl 2-(4-forMylphenoxy)acetate
methyl 2-(4-methanoylphenoxy)ethanoate
2-(4-formylphenoxy)acetic acid methyl ester
acetic acid, (4-formylphenoxy)-, methyl ester
Acetic acid, 2-(4-formylphenoxy)-, methyl ester
物理化学性质
制备方法
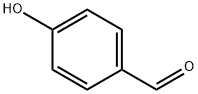
123-08-0
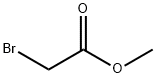
96-32-2

73620-18-5
以对羟基苯甲醛和溴乙酸甲酯为原料合成(4-甲酰基苯氧基)乙酸甲酯的一般步骤:向4-羟基苯甲醛(3.0 g,25 mmol)的丙酮(61.4 mL)溶液中加入K2CO3(5.43 g,39.3 mmol),剧烈搅拌混合物。随后加入溴乙酸甲酯(2.8 mL,29 mmol),并将混合物在室温下搅拌3.5小时。反应完成后,减压浓缩反应混合物,然后用110 mL水洗涤,用乙酸乙酯(EtOAc)萃取。分离有机层,用无水Na2SO4干燥,过滤并浓缩,得到无色油状物,该油状物在真空下固化为白色固体,分离产率为82%。产物经1H NMR(400 MHz,CDCl3)表征:δ 9.87(s, 1H),7.82(d, J = 8.8 Hz, 2H),6.98(d, J = 8.7 Hz, 2H),4.70(s, 2H),3.79(s, 3H)。ESI-MS(m/z):195.1 [M + H]+。
参考文献:
[1] Organic Letters, 2011, vol. 13, # 20, p. 5656 - 5659
[2] Organic Letters, 2004, vol. 6, # 12, p. 2019 - 2022
[3] Chemistry - A European Journal, 2008, vol. 14, # 19, p. 5812 - 5819
[4] Patent: WO2015/65716, 2015, A1. Location in patent: Paragraph 00490
[5] Patent: WO2016/144958, 2016, A1. Location in patent: Paragraph 00296
