74733-29-2
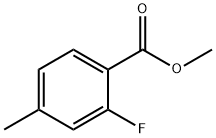 74733-29-2 结构式
74733-29-2 结构式
基本信息
2-Fluoro-4-Methyl-benzoic acid Methyl ester
物理化学性质
制备方法

67-56-1
7697-23-6

74733-29-2
(f)2-氟-4-甲基苯甲酸甲酯(5):方法A:在搅拌下,将2-氟-4-甲基苯甲酸(2.0g,13.0mmol)和K2CO3(3.6g,26.0mmol)的乙腈(15mL)混合物中逐滴加入CH3I(1.6mL,25.6mmol)。反应混合物加热回流过夜后,减压蒸馏除去溶剂。残余物用EtOAc溶解,依次用水和饱和食盐水洗涤,无水Na2SO4干燥,过滤后减压浓缩。粗产物通过硅胶柱色谱纯化,洗脱剂为己烷/EtOAc(6:1),得到白色固体5(1.73g,收率79%),熔点51-53℃。1H NMR(CDCl3)δ:7.83(t,J=8.0Hz,1H),7.00(dd,J=8.0,1.0Hz,1H),6.95(d,J=12.0Hz,1H),3.91(s,3H),2.39(s,3H)。方法B:在搅拌下,将2-氟-4-甲基苯甲酸(7.0g,45.4mmol)的MeOH(100mL)溶液中逐滴加入浓硫酸(5mL)。反应混合物加热回流过夜后,减压蒸馏除去溶剂。残余物用EtOAc溶解,依次用饱和NaHCO3水溶液和饱和食盐水洗涤,无水Na2SO4干燥,过滤后减压浓缩。粗产物通过硅胶柱色谱纯化,洗脱剂为己烷/EtOAc(6:1),得到白色固体5(6.88g,收率90%)。分析数据与方法A相同。
参考文献:
[1] Patent: WO2010/116270, 2010, A1. Location in patent: Page/Page column 55
[2] Organic Process Research and Development, 2011, vol. 15, # 3, p. 565 - 569
[3] Bioorganic and Medicinal Chemistry Letters, 2014, vol. 24, # 7, p. 1742 - 1747
[4] Bioorganic and Medicinal Chemistry Letters, 2015, vol. 24, # 7, p. 1742 - 1747
[5] Patent: EP1449844, 2004, A1. Location in patent: Page 22
