76315-01-0
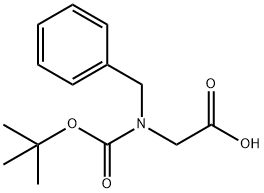 76315-01-0 结构式
76315-01-0 结构式
基本信息
N-苄基-N-叔丁氧羰基甘氨酸
N-Boc-N-benzyl-glycine
N-BENZYL-N-(TERT-BUTOXYCARBONYL)GLYCINE
N-α-(t-Butoxycarbonyl)-N-α-benzylglycine
2-(BENZYL(TERT-BUTOXYCARBONYL)AMINO)ACETIC ACID
(N-benzyl-N-tert-butoxycarbonyl)aminoacetic acid
2-(Benzyl(tert-butoxycarbonyl)amino)acetic acid 95+%
Glycine, N-[(1,1-dimethylethoxy)carbonyl]-N-(phenylmethyl)-
tert-Butyl N-(2-hydroxy-2-oxoethyl)-N-(phenylmethyl)carbamate
物理化学性质
制备方法

24424-99-5
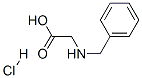
7689-50-1

76315-01-0
以N-苄基甘氨酸盐酸盐(3g,14.88mmol)和二碳酸二叔丁酯(3.25g,14.88mmol)为原料,在二氯甲烷(40mL)中反应。向反应体系中加入三乙胺(6.22mL,44.6mmol),室温搅拌6天。反应完成后,将反应混合物用二氯甲烷和稀盐酸分配,有机相用二氯甲烷(3×20mL)萃取。合并有机层,依次用水(20mL)和盐水(20mL)洗涤,无水硫酸镁干燥。浓缩后得到目标产物N-BOC-N-苄基甘氨酸(5g,125%收率),为透明无色油状物,含有少量三乙胺盐酸盐杂质。产物经1H NMR(400MHz,CDCl3)表征:δ1.38-1.46(d,9H),3.71(s,1H),3.86(s,1H),4.54(d,J=12.38Hz,2H),7.07-7.37(m,5H)。产物无需进一步纯化,可直接用于下一步反应。
参考文献:
[1] Patent: WO2008/76805, 2008, A2. Location in patent: Page/Page column 127
[2] European Journal of Medicinal Chemistry, 2015, vol. 90, p. 547 - 567
[3] Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1997, # 11, p. 1681 - 1690
[4] Patent: WO2007/58862, 2007, A2. Location in patent: Page/Page column 65